| [1] | Flore de Madagascar et des Comores, 1952. Plantes vasculaires. Fam. Moracées, P: 24-29. |
| [2] | Jumelle, 1920. C.R. Acad. Sci., CLXXI, p: 924. |
| [3] | Leandri, 1948. Not. Syst., XII, p: 172. |
| [4] | Decne, 1847. Ann. Sci. Nat. 3ème sér, VIII, p: 108. |
| [5] | Akpata, MI et OE Miachi 2001. Aspects nutritifs de deux plantes alimentaires: Une étude comparative préliminaire. Électronique J. Environ. Agric. Food Chem, 10: 2019-2025. |
| [6] | AOAC (Association of analytical chemists), 1970. Oficials methods of analysis, Association of analytical chemists, Washington, DC USA, USA. |
| [7] | Antia B.S., Akpan E.J., Okon P.A., Umoren I.U, 2006. Nutritive and Anti-Nutritive Evaluation of Sweet Potatoes (Ipomoea batatas) Leaves. Pakistan Journal of Nutrition, 5 (2), 166-168. |
| [8] | Cheftel J-C., Cheftel H. 1977. Introduction à la biochimie et à la technologie des aliments. Volume 1. Technique et Documentation -Lavoisier, Paris, p. 383. |
| [9] | Cozzone A., Bursson F. 1970. Electrophorèse en gel de Polyacrylamide des protéines de S. plantesis et de S. gitleri. C.R.hebd. Séanc-Acad SC. Paris. |
| [10] | Dubois M., Gilles K.A., Hamilton J.K., Roben F. A.et al. 1956. Colorimetric method for determination of sugar and related substances. Anal. Chem, 28, 350-356. |
| [11] | Devani M.B., Shioshoo J.C., Suhagia B.N. 1989. Spectrophotometrical method for microdetermination of nitrogen in Kjeldahl digest. J. Ass. OFMF. Anal. Chem, 72 (6), 953-956. |
| [12] | Fenwick D.E., Oakenfull D. 1983. Saponin content of food plants and some prepared foods. J. Sci Food Agric, 34, 186-191. |
| [13] | Francis G., Kerem Z., Makkar H.P.S., Becker K. 2002. The biological action of saponins in animal systems: a review. British Journal of Nutrition, 88, 587–605.34. Guggenbühl N. Diététicien Nutritionniste. |
| [14] | Fischer E. H., Stein E.A. 1961. DNS colorimetric determination of available carbohydrates in foods. Biochemical Preparation, 8, 30-37. |
| [15] | Goni I., Garcia-Diz L., Manas E., Saura-Calixto F. 1996. Analysis of resistant starch: a method for foods and food products. Food Chemistry, 56, 445–449. |
| [16] | Gupta K., Barat G.K., Wagle D.S., Chawla H.K.L. 1989. Nutrient contents and antinutritional factors in conventional and non-conventional leafy vegetables. Food Chemistry, 31, 105-116. |
| [17] | Hercberg S. 1994. Fer, vitamines, oligo-éléments. I. Le fer. In Enseignement de la nutrition, tome 1, p. 121-131. |
| [18] | Koziol M.J. 1990. Afrosimetrie Estimation of Threshold Saponin Concentration for Bitterness in Quinoa. Journal of the Science of Food and Agriculture, 54 (2), 211-220. |
| [19] | Lehninger A.L. 1982. La nutrition humaine. In Principe de biochimie. Edition Flammarion Médecine Sciences, pp. 753-789. |
| [20] | Marigo G. 1973. Méthode de fractionnement et d’estimation des composés phénoliques chez les végétaux. Analysis, 2 (2), 106-110. |
| [21] | Noonan S.C., Savage G.P. 1999. Oxalate content of food and its effect on humans. Asia Pacific Journal of Clinical Nutrition, 64-74. |
| [22] | Olesek W. et al. 2001. Steroidal saponins of Yucca schidigera Roezl. J. Agric. Food. Chem, 49(9), 4392-4396. |
| [23] | Parke D.V., Ioannides C. 1981. The role of nutrition in toxicology. Ann. Rev. Nutr, 1, 207-234. |
| [24] | Pingle, U. et BV Ramastin 1978. Analyse chimique des aliments. .. 7 EDN, Church Hill Livingstone, Londres, Royaume - Uni, pp: 72-73,138-143, 488-496. |
| [25] | Rouers B. 1996. L'eau, agent de détoxication alimentaire Étude de deux techniques de détoxication des plantes alimentaires utilisées par les Aborigènes Australiens. Altérité, 1(1). |
| [26] | FONG et coll., 1974, en utilisant des réactifs chimiques spécifiques. |
| [27] | Abdullahi SA, Abdullahi GM. 2005. Effect of Boiling on the Proximate, Anti-Nutrients and Amino Acid Composition of Raw Delonix regia Seeds. Niger. Food J. 23: 128-132. |
| [28] | Adewusi SRA, Falade OS. 1996. The Effect of Cooking on extractable tannin, phytate, sugars and mineral solubility in some improved Nigerian Legume Seeds. Food Sci. Technol. Int. 2: 231-240. |
| [29] | Association of Official analytical Chemists (AOAC). 1984. Official Methods of Analysis 14th Edition. |
| [30] | Barker MM. 1996. Nutrition and Dietics for Health Care.9th Edn. Churchill Livingston New York, N.Y., pp. 92-101. |
| [31] | Baumer M (1995). Food producing trees and shrubs of West Africa. Serie- Etudes –et Recherches, Senegal pp. 168-260. |
| [32] | Dreon DM, Vranizan KM, Krauss RM, Austin MA, Wood PD. 1990. The effects of polyunsaturated fat and monounsaturated fat on plasma, Lipoproteins. J. Am. Med. Assoc. 263: 2462. |
| [33] | Elias LG, De Fernandez DG, Bressani R. 1979. Possible effects of seed coat Polyphenolics on the Nutritional Quality of Bean Protein. J. Food Sci. 44(2): 524-526. |
| [34] | Eromosele IO, Eromosele CO, Kuzhkuzha DM. 1991. Evaluation of mineral elements and ascorbic acid contents in fruits of some wild plants. Plant Hum. Nutr. 41: 151-154. |
| [35] | Eromosele IC, Eromosele CO. 1993. Studies on the chemical composition and physio-chemical properties of seeds of some wild plants: (Netherland) Plant Food Hum. Nutr. 43: 251-258. |
| [36] | Food and Nutrition Board (FNB). 1974. Recommended dietary allowances. 8th edition National Academy of Sciences, National Research Council, Washington D.C. Harland BF. |
| [37] | Oberleas D. 1986. Anion exchange method for determination of phytates in food: collaborative study. J. Assoc. Off. Anal. Chem. 69: 667-670. |
| [38] | Kakade ML, Rackis JJ, Mc Ghee JE, Puski G. 1974. Determination of trypsin Inhibitor activity of soy products: A collaborative analysis of an improved procedure. Cereal Chem. 51: 376-383. |
| [39] | Liener IE, Kakade ML. 1980. Proteaseinhibitors. In: Liener I (ed).Toxic constituents of plant food stuffs, second edition, New York, Academic Press, pp. 7-71. |
| [40] | Liener IE. 1994. Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 34: 31-67. |
| [41] | Munro A, Bassir O. 1989. Oxalate in Nigerian vegetables. W. Afr. J. Biol. Appl. Chem. 12: 14-18. |
| [42] | Olaofe O, Akogun OO. 1990. Mineral and Vitamin C content and their distribution in some fruits. Niger. Food J. 8: 111. |
| [43] | Price ML, Scoyoc SV, Butler LG. 1978. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 26: 1214-1218. |
| [44] | Reddy MB, Love M. 1999. The impacts of food processing on the nutritional quality of vitamins and minerals. Adv. Exp. Med. Biol. 459: 99-106. |
| [45] | Thompson LU. 1993. Potential health benefits and problems associated with anti nutrients in foods. Food Res. Intl. 26: 131-149. |
| [46] | Umoh IB. 1998. Commonly used fruits in Nigeria. In: Nutritional Quality of Plant Foods. (Eds Osagie AU, Eka OU). Post harvest Research Unit, University of Benin, Benin city. Nigeria. |
| [47] | Zarkada CG, Voldeng HD, Vu UK. 1997. Determination of the protein quality of three new northern adapted cultivars or common and mico types soya beans by amino acids. |



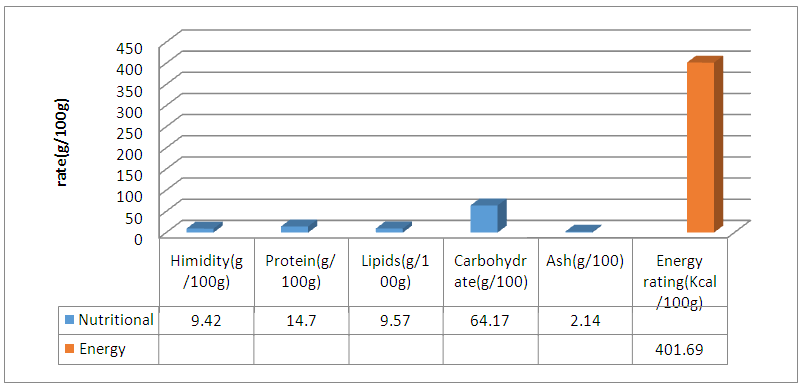
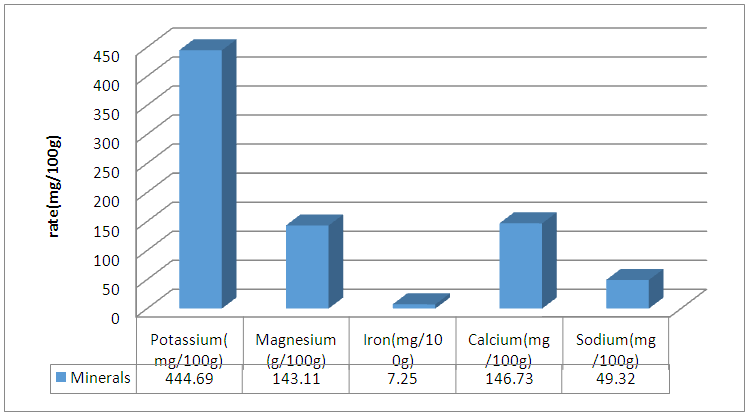
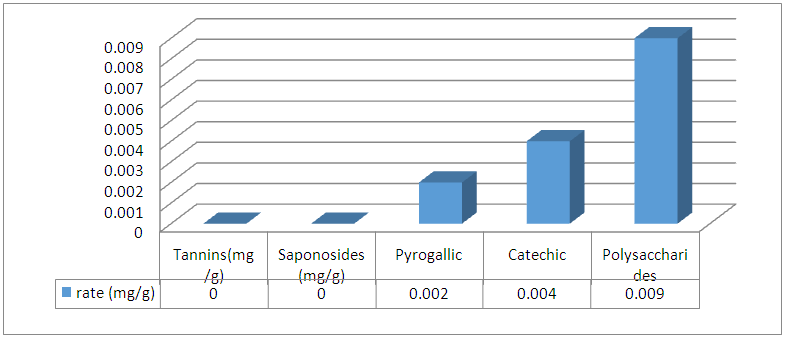
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML