-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(4): 75-90
doi:10.5923/j.food.20170704.03

Development of Spiced Instant ‘Moinmoin’ Produced from Precooked Cowpea Flour using Maize Starch as Binder
Olu. Malomo, Rotimi Apo, Emmanuel Adediran Alamu
Bells University of Technology/ Corinthian Spices, Ota, Nigeria
Correspondence to: Olu. Malomo, Bells University of Technology/ Corinthian Spices, Ota, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The cowpea flour samples were produced from de-hulled cowpea seeds and pre-cooked de-hulled cowpea seeds, and were spiced. The flour from the former was used as the control while the flour from the latter was used as the experimental samples. The pre-cooked flour was divided into 4 portions and cornstarch (the binder) was added at (0%, 10%, 15% and 20%) varying levels. Resultant flours were analyzed for proximate composition, functional and pasting properties, some minerals, anti-nutritional factors and amino acids index to compare with the control. The flours were also used to prepare ‘moin-moin’ (steamed cowpea paste) to compare their cooking time properties with the control as well. The results of the study indicated a significant decrease (P ≤ 0.05) in protein, oil absorption, bulk density, peak viscosities and amino acids index and ranged from (23.94 – 19.24%; 1.90 – 1.45ml/g; 0.74 – 0.65g/ml; 371.70 – 294.92RVU and 0.232 – 0.007) respectively. Anti-nutritional factors measured, decreased significantly (P ≤ 0.05) for phytates, saponin, oxalate and Trypsin inhibitors (5.79 – 2.56%; 8.27 – 5.03%; 10.77 – 4.75% and 0.47 – 0.21%) respectively. On the other hand the pasting properties, analysed as peak viscosities, minerals and amino acids index of the pre-cooked samples increased significantly (P ≤ 0.05) with increasing addition of the maize binder. The carbohydrate components increased significantly (P ≤ 0.05) and ranged from 58.74 – 63.95%.
Keywords: ‘Moinmoin’, Oil absorption, Bulk density, Anti-nutritional factors, Trypsin inhibitors, Amino acid index, and pasting property- peak viscosity
Cite this paper: Olu. Malomo, Rotimi Apo, Emmanuel Adediran Alamu, Development of Spiced Instant ‘Moinmoin’ Produced from Precooked Cowpea Flour using Maize Starch as Binder, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 4, 2017, pp. 75-90. doi: 10.5923/j.food.20170704.03.
Article Outline
1. Introduction
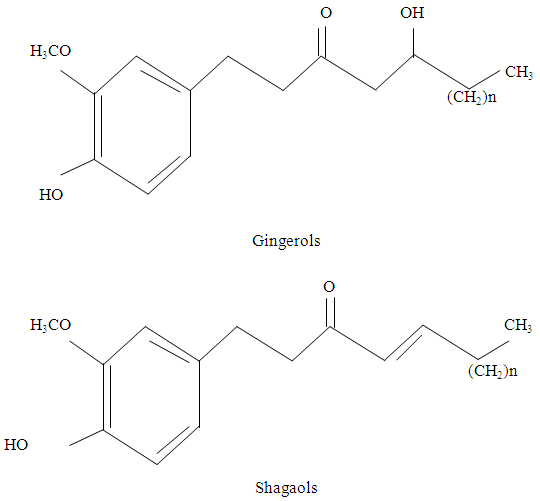
- Cowpea (Vigna unguiculata), commonly known as grain legume is widely distributed in tropical and sub-tropical countries, covering Africa, Asia, Southern Europe and Central South America (Davis, 2013). It is one of the ancient crops known to man and is cultivated primarily for grain, but also as vegetable (leafy green, green pods, shelled dried peas, and fresh shelled green peas), a folder and cover crop. According to I.I.T.A (2012), cowpea dates back to the Ancient West African Cereal farming where its cultivation is associated with that of Sorghum and Millet.Cowpea is a drought tolerant and warm weather crop. It is well adapted to drier regions of the tropics. It grows well in poor soils, with more than 85% sand and with less than 0.2% organic matter and low levels of phosphorus (Manjula, 2011). Musa (2010) report that with the use of improved technologies, yield of 1,500 – 2000kg/ha can be obtained on sole cropping system. Efficiency and productivity potentials are also high if the farmers use more of improved seeds, family labour, agrochemicals, less hired land and labour (Jirgi, 2010). Its deep penetrating root system enables it to withstand very dry conditions. Legume crops because of their Nitrogen-fixing trait play important roles in conservation farming systems and contribute to food security in the developing world. (Daryanto et al; 2015).In Nigeria and in many African countries, cowpea is an important, nutritious leguminous crop, providing an alternative source to animal protein (Dolvo et al; 1976).They are equally important both in human and animal nutrition especially in tropical-Africa where they are more consumed (Burkill, 1995).Cowpea has found utilization in various ways in traditional and modern food processing in the world. The seed of cowpea can be cooked in the dried form, sprouted, or ground into flour, an intermediate product (Dolvo et al; 1976). Cowpea is also consumed in the form of bean pudding, ‘akara’ (fried cowpea paste); moin-moin (steamed cowpea paste). Bean soup (traditionally known as ‘gbegiri’) can also be prepared from cowpea. Bean soup is eaten with reconstituted yam flour product- “amala.” Legumes are multipurpose crops. At the household, cottage and large scale levels, flours have been processed from different types of legumes. Due to changing trends in consumer demands for more convenient products, cowpea flour has added to household convenience (Ashaye et al; 2000; Fasoyiro, et al; 2010).The common unit operations involved in flour production include washing, soaking, de-hulling, drying, milling, sieving and packaging. Flours have been developed into different household recipes such as cake, cookies, ‘kokoro’ (Granito, et al; 2010; Omueti and Morton, 1996) with comparable sensory attributes with products from freshly prepared legumes. Composite flours have also been developed from cereals and tuber crops mixed with legume flours. Cowpea flour is usually rehydrated and utilized in formulations as desired.However, the growth in the dietary share of cowpea has been constrained by high preparation time, labour requirements and undesirable product characteristics including beany flavour, low digestibility and abdominal upset as well as post-harvest grain losses to insect pests (Dolvo et al; 1976; McWatters, 1983; Henshaw and Lawal, 1993).Protein-energy malnutrition called ‘Kwashiokor’ (condition of impaired development or function caused by either a long-term deficiency or an excess in nutrient intake). While ‘marasmus’ – ‘to-waste-away’ (condition where infants slowly starving to death). Majority of brain growth occurs between conception and first birthday; highest growth rate is at birth. If diet does not support this process, it may not grow to its full size. This reduced or retarded growth may lead to diminished intellectual function.Both ‘kwashiorkor’ and ‘marasmus’ wreak havoc on infants and children in developing countries. Mortality rate is approximately 10 to 20 times higher in developing countries than in developed countries as shown in the data below. According to Wardlaw (2003), recommended dietary allowance (RDA) for adult is 0.8g/kg body weight/day. This translates to: Men = 56g protein/day for a 70kg manWomen = 44g protein/day for a 55kg womanChildren need proportional greater requirement because they are growing and developing. Also, greater requirement is needed during pregnancy and lactation period in women.High quality protein mainly from animal sources such as meat, fish and poultry are also rich in fat. Excess fat are linked to cardiovascular diseases. High protein diets also breaks down the pancreas and lowers resistance to cancer, arthritis, osteoporosis and promotes aging as reported by Dye (1999). This is prevalent among the affluent.Therefore, to both the rich and the poor, cowpea protein presents a more health-supportive mix of protein-fiber than any other group of food commonly eaten world-wide.Fox and Cameron (1995), underscoring the importance and value of plant proteins reported and advised to go directly to the plant since most mixed plant diets provided adequate supply of protein. Conversion of plants into animal protein in cattle and other animals is extremely inefficient and extravagant use of world’s food resources. Plant foods provide energy, protein, magnesium and dietary fiber. They don’t contain cholesterol, rather abundant unsaturated fatty acids that do not raise blood cholesterol as does saturated fats. In cowpea, methionine is the limiting amino acid (Elegbede, 1988). The limiting amino acid may lead to poor utilization of amino acid by humans so that relatively more protein is required to meet the minimum requirement for protein synthesis.Common Spices in Nigerian Dishes and SnacksPreparation of many Nigerian dishes usually require culinary; herbs and spices which consists of ingredients such as onions, pepper, ginger, locust beans, garlic, turmeric, nutmeg etc. (Alabi, 2007). Herbs and spices are integral parts of the daily diet; and can add variety, flavour, colour and aroma to the everyday diet whilst contributing a wide range of both nutrients and bioactive that may contribute to improved health (Murphy et al; 1978; Kitts, 1994). Herbs and spices may act synergistically to enhance the health-related properties of other foods (Thimayamma et al; 1983). Flavours and seasonings are important considerations for snacks and could be used as both flavourings and functional ingredients in snack products (Williams, 1999; Pszczola, 1999).Dietary spices influence various systems in the body such as gastrointestinal, cardiovascular, and reproductive and nervous systems resulting in diverse metabolic and physiologic actions (Kochhar, 2008). Any good spice should be convenient to consume, inexpensive, nutritious, low in fat and have a long shelf life. Three from among the many spices commonly found in Nigeria used in the preparation of ‘moin-moin’ are namely; Ginger, Onions and Pepper.GINGER (Zingiber officinale)Ginger has many therapeutic attributes such as antimicrobial, anti-inflammatory and anti-cancer activity (Ohaeri and Adoga, 2006). Ginger is a mixture of over several hundred known constituents including gingerols, shagaols, β-carotene, caffeic acid, curcumin, ealicylate and capsaiun (Schulick, 1996). Ginger owes its characteristic organoleptic properties to two classes of constituents. The aroma of ginger are due to the constituents of its steam-volatile oil which are mainly sesquiterpence hydrocarbons, monoterpence hydrocarbons and oxygenated monoterpenes (Purseglove et al 1981) while its pungency is due to the non-steam volatile components also known as the gingerols.Ginger is a major tranquilizer due to its gingerol. It is used as a spice as well as an important medicinal product. The responsible constituents are believed to be gingerols and shagaols (Phillips et al. 1993) as shown below:Major constituents of ginger are shown below
 Onion (Allium cepa)Onion and its juice may be used to treat appetite loss, prevention of age-related changes in blood vessels, minor digestive disturbances (VanWyk and Wink, 2005). Onions undergo enzymatic breakdown of sulphur-containing substances due to damages of tissue to give pungent volatiles that cause weeping (VanWyk, 2005).The pharmacological activity as well as the pungent smell are due to the several sulphur-containing compounds mainly sulphoxides such as trans-5-(1-propenyl)-L-(+)- Cysteine Sulphoxides) and cepaenes (α-sulphinyl-disulphides) as shown below: (VanWyk and Wink, 2005).
Onion (Allium cepa)Onion and its juice may be used to treat appetite loss, prevention of age-related changes in blood vessels, minor digestive disturbances (VanWyk and Wink, 2005). Onions undergo enzymatic breakdown of sulphur-containing substances due to damages of tissue to give pungent volatiles that cause weeping (VanWyk, 2005).The pharmacological activity as well as the pungent smell are due to the several sulphur-containing compounds mainly sulphoxides such as trans-5-(1-propenyl)-L-(+)- Cysteine Sulphoxides) and cepaenes (α-sulphinyl-disulphides) as shown below: (VanWyk and Wink, 2005).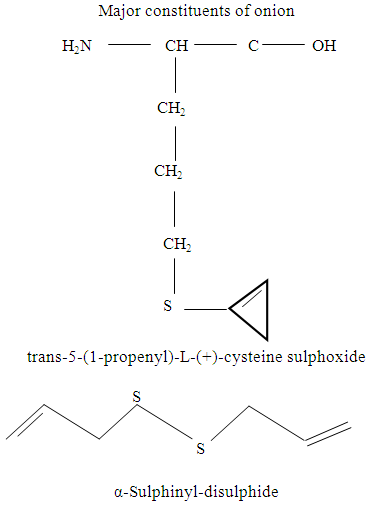 RED PEPPER (Capsicum annum)Red pepper produces capsain and capsaicin used as spice and medicine (Columbus, 1978). Capsaicin, the pungent active principle of red chilli has been shown to cause gastric mucosal oedema and hyperemia and decrease in the gastric acid output (Desai et al; 1977).
RED PEPPER (Capsicum annum)Red pepper produces capsain and capsaicin used as spice and medicine (Columbus, 1978). Capsaicin, the pungent active principle of red chilli has been shown to cause gastric mucosal oedema and hyperemia and decrease in the gastric acid output (Desai et al; 1977).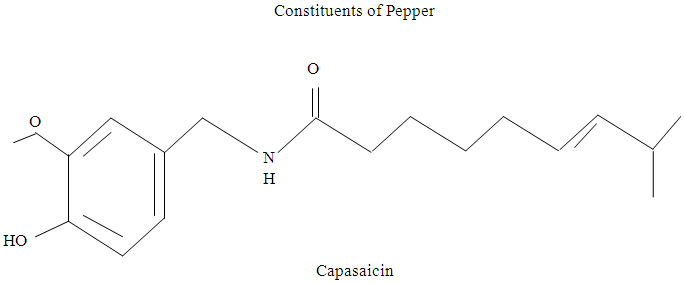 Gelatinization in FoodsSwelling and gelatinization in cowpea flour with all starches, leaching of polysaccharides (amylose and/or amylopectin), depending on the starch was highly correlated with swelling factor and that swelling is a property of the amylopectin (Atwell et al; 1988).Gelatinization in the narrowest sense is the thermal disordering of crystalline structures in native starch granules, but in the broader sense it includes related events such as swelling of the granules and leaching of soluble polysaccharides (Atwell et al; 1988). However, in most food systems, the actual temperature at which starch gelatinizes is less important than those properties that depend on swelling, such as pasting behaviour and rheological properties of the practically or fully swollen starch granules. The properties of the starch water system will of course be different if the swollen granules are dispersed mechanically to give a uniform gel.Gelatinization and Pasting Properties of CowpeaPasting properties are functional properties relating to the ability of an item to act in paste-like manner. Pasting characteristics have been associated with cooking and textural quality of various food products (Otegbayo et al; 2006).When starch based foods are heated in aqueous environment, they undergo series of changes known as gelatinization and pasting. These are two of the most important properties that influence quality and aesthetic consideration for food systems, since they affect texture and digestibility as well as the end use of starchy foods (Adebowale et al; 2005).Pasting properties of cowpea flour can be determined using Rapid Visco Analyzer (RVA) to obtain the following parameters: Peak, trough and cooled paste viscosities, which can then be used to determine the consistency, set back and break down, as well as the pasting temperature and time.Pasting temperature is the temperature where viscosities first increase by at least 2RVU (Rapid Visco Units) over a 20sec period; and it gives an indication of the minimum temperature required to cook the flour (Olkku and Rha, 1978).Peak viscosity is the maximum viscosity developed during or soon after the heating portion of the test. Viscosity increased rapidly after gelatinization because of associated further disintegration of the granules at elevated temperature. Degrees of viscosities were indicative of various degrees of starch gelatinization (Dengate, 1984).Basic Ingredients in ‘Moin-moin’ Preparation and Quality‘Moin-moin’ (Steamed cowpea paste) is a food traditionally prepared from de-hulled and wet-milled seeds (Henshaw et al; 2009). It is a Nigerian steamed bean pudding made from a mix of washed and peeled beans, onions and fresh ground peppers (Usually a combination of bell peppers and chilli or scotch bonnet).According to Akusu and Kiin-Kabari (2012), ‘moin-moin’ is a gel produced by heating slurries containing cowpea solids of 15% and above. Cowpea paste is obtained by wet-milling of the de-hulled beans or by mixing cowpea flour with water and small amounts of vegetable oil and other ingredients to form a homogenous slurry or paste. On heating the slurry in pouches made from leaves or Aluminum foil, cooking in boiling water or steam, it solidifies into an irreversible gel between 73-87°C (Okechukwu et al; 1992).Typical ‘moin-moin’ recipe:5g/100g of ground bell pepper (colour, flavour, taste)2.5ml/100g paste of ground onions (taste, flavour)35ml/100g of vegetable oil (smoothness, mouth feel)2.5g/100g of salt (taste, flavour) The slurry is light-coloured, having starch and protein which are desirable. When cooked, it is brightly orange-coloured in appearance (Prinyawiwatkul and McWatters, 1996). It has a mild texture with characteristically smooth and moisty mouth feel.
Gelatinization in FoodsSwelling and gelatinization in cowpea flour with all starches, leaching of polysaccharides (amylose and/or amylopectin), depending on the starch was highly correlated with swelling factor and that swelling is a property of the amylopectin (Atwell et al; 1988).Gelatinization in the narrowest sense is the thermal disordering of crystalline structures in native starch granules, but in the broader sense it includes related events such as swelling of the granules and leaching of soluble polysaccharides (Atwell et al; 1988). However, in most food systems, the actual temperature at which starch gelatinizes is less important than those properties that depend on swelling, such as pasting behaviour and rheological properties of the practically or fully swollen starch granules. The properties of the starch water system will of course be different if the swollen granules are dispersed mechanically to give a uniform gel.Gelatinization and Pasting Properties of CowpeaPasting properties are functional properties relating to the ability of an item to act in paste-like manner. Pasting characteristics have been associated with cooking and textural quality of various food products (Otegbayo et al; 2006).When starch based foods are heated in aqueous environment, they undergo series of changes known as gelatinization and pasting. These are two of the most important properties that influence quality and aesthetic consideration for food systems, since they affect texture and digestibility as well as the end use of starchy foods (Adebowale et al; 2005).Pasting properties of cowpea flour can be determined using Rapid Visco Analyzer (RVA) to obtain the following parameters: Peak, trough and cooled paste viscosities, which can then be used to determine the consistency, set back and break down, as well as the pasting temperature and time.Pasting temperature is the temperature where viscosities first increase by at least 2RVU (Rapid Visco Units) over a 20sec period; and it gives an indication of the minimum temperature required to cook the flour (Olkku and Rha, 1978).Peak viscosity is the maximum viscosity developed during or soon after the heating portion of the test. Viscosity increased rapidly after gelatinization because of associated further disintegration of the granules at elevated temperature. Degrees of viscosities were indicative of various degrees of starch gelatinization (Dengate, 1984).Basic Ingredients in ‘Moin-moin’ Preparation and Quality‘Moin-moin’ (Steamed cowpea paste) is a food traditionally prepared from de-hulled and wet-milled seeds (Henshaw et al; 2009). It is a Nigerian steamed bean pudding made from a mix of washed and peeled beans, onions and fresh ground peppers (Usually a combination of bell peppers and chilli or scotch bonnet).According to Akusu and Kiin-Kabari (2012), ‘moin-moin’ is a gel produced by heating slurries containing cowpea solids of 15% and above. Cowpea paste is obtained by wet-milling of the de-hulled beans or by mixing cowpea flour with water and small amounts of vegetable oil and other ingredients to form a homogenous slurry or paste. On heating the slurry in pouches made from leaves or Aluminum foil, cooking in boiling water or steam, it solidifies into an irreversible gel between 73-87°C (Okechukwu et al; 1992).Typical ‘moin-moin’ recipe:5g/100g of ground bell pepper (colour, flavour, taste)2.5ml/100g paste of ground onions (taste, flavour)35ml/100g of vegetable oil (smoothness, mouth feel)2.5g/100g of salt (taste, flavour) The slurry is light-coloured, having starch and protein which are desirable. When cooked, it is brightly orange-coloured in appearance (Prinyawiwatkul and McWatters, 1996). It has a mild texture with characteristically smooth and moisty mouth feel.2. Materials and Methods
- Basic Raw MaterialsŸ Cowpea seeds (Vigna unguiculata)Ÿ Spices (Dried/ground onions, pepper, ginger and table salt)Processing Equipment/Machinery and accessories(Available in Esculent Race Foods Ltd, Oyo State Nigeria)
 Analytical Equipment (Available in Food and Central Laboratories, Bells University of Technology, Ota, Ogun State, Nigeria)
Analytical Equipment (Available in Food and Central Laboratories, Bells University of Technology, Ota, Ogun State, Nigeria) RAW MATERIALS SOURCINGThe brown variety of cowpea seeds (Vigna unguiculata) and the spices (onions, peppers-‘shombo’, ginger) used for this study were purchased from a local market in Oyo State, Nigeria.PREPARATION OF COWPEA FLOUR.The cowpea flour was prepared according to the method (modified) described by Okaka (1997) as shown in figures 1 and 2. Ten kilograms of cowpea seeds were weighed after cleaning, sorting and grading. Cleaned seeds were then soaked in portable water for 10-15 minutes and de-hulled using a locally fabricated de-huller. After draining, de-hulled seeds were divided into two portions of 2 kilograms and 8 kilograms.
RAW MATERIALS SOURCINGThe brown variety of cowpea seeds (Vigna unguiculata) and the spices (onions, peppers-‘shombo’, ginger) used for this study were purchased from a local market in Oyo State, Nigeria.PREPARATION OF COWPEA FLOUR.The cowpea flour was prepared according to the method (modified) described by Okaka (1997) as shown in figures 1 and 2. Ten kilograms of cowpea seeds were weighed after cleaning, sorting and grading. Cleaned seeds were then soaked in portable water for 10-15 minutes and de-hulled using a locally fabricated de-huller. After draining, de-hulled seeds were divided into two portions of 2 kilograms and 8 kilograms.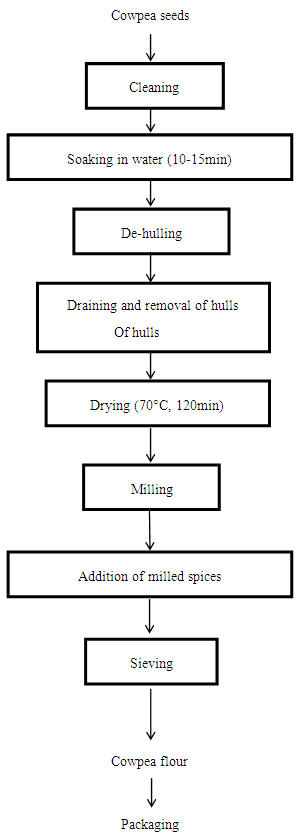 | Figure 1. Flow chart for the production of cowpea flour |
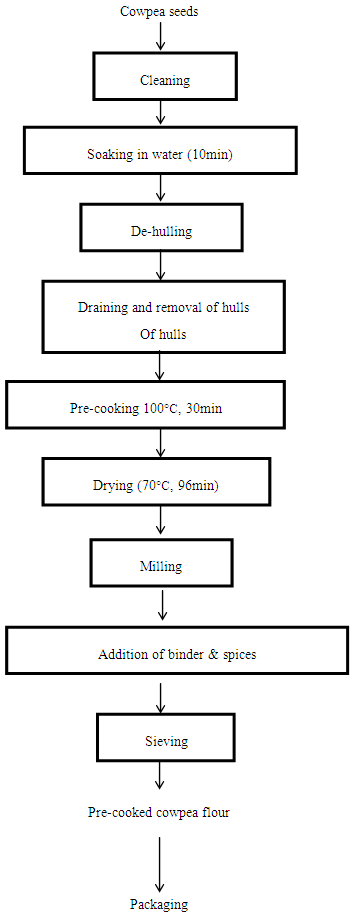 | Figure 2. Flow chart for the production of pre-cooked cowpea flour |
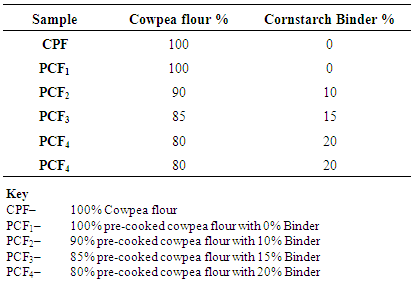 | Figure 3. Blend formulation for cowpea flour and Binder |
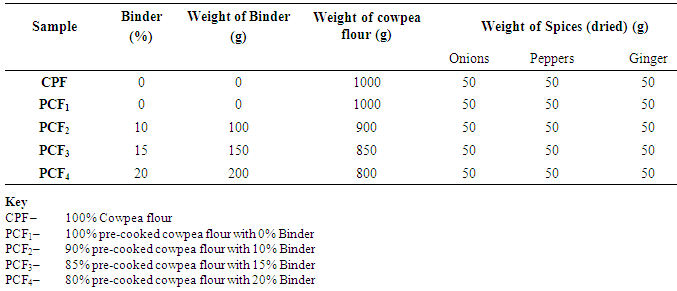 | Figure 4. Blend formulation for the cowpea flours and spices |
3. Methods of Analysis
- Proximate Composition Determination of the Cowpea floursDetermination of proximate composition; moisture, crude fat, crude protein and ash were determined using standard methods as described by (AOAC, 2004). The total carbohydrate was obtained by difference.Moisture Content DeterminationApproximately 2g of flour sample was weighed out separately and in triplicates. It was then placed in drying oven at 105°C and dried to a constant weight then cooled in a desiccator; weighed again not to expose sample to the atmosphere.Percentage moisture content (Table 4.1) was calculated using the formula:
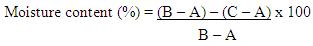 Where:A = Weight of clean dry moisture can (g)B = Weight of clean dry moisture can + wet sample (g)C = Weight of clean dry moisture can + dry sample (g)Crude Fats DeterminationApproximately 2g of flour sample was weighed out separately and in triplicates into a dry extraction thimble and placed in the Foss Soxtec 2055 fats extractor. The fats were extracted from the sample with petroleum ether, and evaluated as a percentage of the weight before the solvent was evaporated.Defatted sample was kept and used in determining crude fiber.Percentage crude fat content (Table 4.1) was calculated using the formula:
Where:A = Weight of clean dry moisture can (g)B = Weight of clean dry moisture can + wet sample (g)C = Weight of clean dry moisture can + dry sample (g)Crude Fats DeterminationApproximately 2g of flour sample was weighed out separately and in triplicates into a dry extraction thimble and placed in the Foss Soxtec 2055 fats extractor. The fats were extracted from the sample with petroleum ether, and evaluated as a percentage of the weight before the solvent was evaporated.Defatted sample was kept and used in determining crude fiber.Percentage crude fat content (Table 4.1) was calculated using the formula: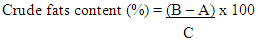 Where:A = Weight of clean dry flask (g)B = Weight of flask with fat (g)C = Weight of sample (g)Ash Content DeterminationApproximately 2g of flour sample was weighed out separately and in triplicates into a clean dry crucible and then placed in a muffle furnace at 550°C and ashed to a constant weight, cooled in a desiccator and weighed again.Percentage ash content (Table 4.1) was calculated using the formula;
Where:A = Weight of clean dry flask (g)B = Weight of flask with fat (g)C = Weight of sample (g)Ash Content DeterminationApproximately 2g of flour sample was weighed out separately and in triplicates into a clean dry crucible and then placed in a muffle furnace at 550°C and ashed to a constant weight, cooled in a desiccator and weighed again.Percentage ash content (Table 4.1) was calculated using the formula;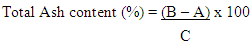 Where:A = Weight of clean dry crucible (g)B = Weight of crucible samples (g)C = Weight of sample (g)Crude Fiber The defatted sample was used in determining the crude fiber. The sample was digested in H2SO4 and NaOH solutions and the residue calcined. The difference in weight after calcination indicated the quantity of fiber present.(i) Defatted dry ample was weighed and placed in the flask (200ml).(ii) 80ml of H2SO4 was added and boiled for 30 minutes.(iii) It was filtered and the residue transferred to the flask, 80ml of NaOH added and boiled for 30 minutes.(iv) Hydrolyzed mixture carefully filtered.(v) Residue washed with distilled water. Washing finished off with three washes of petroleum ether.(vi) Residue was placed in a clean dry crucible and weighed.(vi) The crucible + residue were placed in a muffle furnace at 550°C and ashed to a constant weight.(vii) Cooled in a desiccator and weighed.Percentage of crude fiber content (Table 1) calculated as:
Where:A = Weight of clean dry crucible (g)B = Weight of crucible samples (g)C = Weight of sample (g)Crude Fiber The defatted sample was used in determining the crude fiber. The sample was digested in H2SO4 and NaOH solutions and the residue calcined. The difference in weight after calcination indicated the quantity of fiber present.(i) Defatted dry ample was weighed and placed in the flask (200ml).(ii) 80ml of H2SO4 was added and boiled for 30 minutes.(iii) It was filtered and the residue transferred to the flask, 80ml of NaOH added and boiled for 30 minutes.(iv) Hydrolyzed mixture carefully filtered.(v) Residue washed with distilled water. Washing finished off with three washes of petroleum ether.(vi) Residue was placed in a clean dry crucible and weighed.(vi) The crucible + residue were placed in a muffle furnace at 550°C and ashed to a constant weight.(vii) Cooled in a desiccator and weighed.Percentage of crude fiber content (Table 1) calculated as: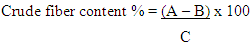 Where:A = Weight of crucible with dry residue (g)B = Weight of crucible with ash (g)C = Weight of sample (g)Crude Protein DeterminationThe Kjeldahl method of protein analysis was used. Approximately 1 g of flour sample was weighed out separately and in triplicates. The sample was first digested using the Foss TecatorTM Digestor, distilled using Kjectec 2200 Distillation Apparatus and titrated using the automated Titre equipment. The blank titre was also carried out and recorded.The percentage Nitrogen was then calculated (Table 4.1) using the formula:
Where:A = Weight of crucible with dry residue (g)B = Weight of crucible with ash (g)C = Weight of sample (g)Crude Protein DeterminationThe Kjeldahl method of protein analysis was used. Approximately 1 g of flour sample was weighed out separately and in triplicates. The sample was first digested using the Foss TecatorTM Digestor, distilled using Kjectec 2200 Distillation Apparatus and titrated using the automated Titre equipment. The blank titre was also carried out and recorded.The percentage Nitrogen was then calculated (Table 4.1) using the formula: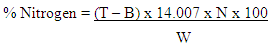 Where: T = Titre value of the sampleB = Titre value of the blank (catalyst + acid = 0.34)N = Normality of acid used in titration (0.1N)The percentage crude protein (Table 1) was then calculated as:
Where: T = Titre value of the sampleB = Titre value of the blank (catalyst + acid = 0.34)N = Normality of acid used in titration (0.1N)The percentage crude protein (Table 1) was then calculated as: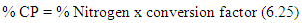 Analysis of Functional PropertiesThese were analyzed according to the methods described by Onwuka (2005) and by Konik et al (1993)Bulk DensityApproximately 2g of the flour sample was weighed out separately and in triplicates, and put into a 10ml measuring cylinder. The cylinder was tapped several times on a laboratory bench to a constant volume. The volume of sample was recorded.
Analysis of Functional PropertiesThese were analyzed according to the methods described by Onwuka (2005) and by Konik et al (1993)Bulk DensityApproximately 2g of the flour sample was weighed out separately and in triplicates, and put into a 10ml measuring cylinder. The cylinder was tapped several times on a laboratory bench to a constant volume. The volume of sample was recorded.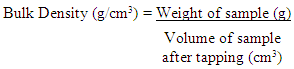 Water Absorption Capacity10ml of distilled water was added to approximately 2g of flour sample in a centrifuge tube. The tube was agitated in a vertex mixer for 2 minutes. It was the centrifuged at 2000r.p.m for 10 minutes. The clear supernatant was decanted and measured to obtain volume of water absorbed. Water absorbed capacity was expressed as volume of water bound by 100g dried flour (ml/g) as shown in Table 4.2.Oil Absorption Capacity10ml of refined vegetable oil was added to approximately 2g of flour sample in a centrifuge tube. The tube was agitated in a vertex mixer for 2 minutes. It was then centrifuged at 2000rpm for 10 minutes. The volume of free oil was decanted and measured to obtain volume of oil absorbed. Oil absorption capacity was expressed as volume of oil bound by 100g dried flour (ml/g) as shown in Table 4.2.Pasting Properties Determination of the Cowpea flour Samples.Rapid Pasting Method Using the Newport Rapid Visco AnalyzerPasting characteristics were determined with a Rapid Visco Analyzer (RVA) (Model RVA 3D+, Newport Scientific Australia, (1998). First, 2.5 g of flour sample were weighed into a dried empty canister; then 25 ml of distilled water was dispensed into the canister containing the sample. The solution was thoroughly mixed and the canister was well fitted into the RVA as recommended. The slurry was heated from 50-95°C with a holding time of 2 min followed by cooling to 50°C with 2 min holding time. The rate of heating and cooling were at a constant rate of 11.25°C per min. Peak viscosity, trough, breakdown, final viscosity, set back, peak time and pasting temperature were read from the pasting profile with the aid of thermo cline for windows software connected to a computer (Newport Scientific, 1998). The viscosity was expressed in terms of Rapid Visco Units (RVU), which is equivalent to 10 centipoises. Results obtained are shown in Table 4.3.Analysis of MineralsAnalysis of some Minerals in the Cowpea flour SamplesThe samples were analyzed for some metals using standard method. These include Calcium, Magnesium, Potassium, Sodium and Phosphorous. The results obtained expressed in percentage for the first three and in parts per million (ppm) for the last two as shown in Table 4.4.Sodium and potassium were determined using a flame photometer (corning, UK model 403) using NaCl and KCl to prepare the standards. Phosphorus was determined colorimetrically using spectronic 20 (Gallenkamp, UK) as described by Pearson (1976) with KH2PO4 as the standard. Other metals were determined using atomic absorption spectrophotometer (Perkin-Elmer model 403, walk CT, USA).Concentration of each metal was calculated from a standard graph based on known concentration of the metal.Anti-nutritional Factors in the Cowpea flour SamplesThe samples were analyzed for some anti-nutritional factors such as Phytates, Saponin, Oxalate and Trypsin Inhibitor. Results obtained are shown in Table 4.5.Oxalate determinationTotal oxalate was determined according to Day and Underwood (1986) procedure. To 1gm of flour sample, 75ml of 15N H2SO4 was added. Solution was carefully stirred intermittently with magnetic stirrer for one hour and filtered using Whatman No 1 filter paper. 25ml of the filtrate was then collected and titrated against 0.1N KMnO4 solutions till a faint pink colour appeared that persists for 30 seconds.The concentration of oxalate was calculated from a standard graph based on known concentration of oxalate.Results obtained are shown in table 4.5.Phytate DeterminationPhytate was determined using Reddy and Love (1999) method. 4g of sample was soaked in 100ml of 2% HCl for 5hrs and filtered. To 25ml of the filtered, 5ml of 0.3% ammonium thiocynate solution was added. The mixture was then titrated with Iron (III) chloride solution until a brownish-yellow colour that persisted for 5minutes was obtained. The concentration of phytate was calculated from a standard graph based on known concentration of phytate.Results obtained are shown in table 4.5.Saponins DeterminationThe procedure of Brunner (1984) was used in the determination of saponins. 2g of flour sample was added to 100ml of isobutyl alcohol (octanol) and left on a UDY shaker for 5hours. The mixture was later filtered with No1 Whatman filter paper. Thefiltrate was transferred and was saturated with magnesium carbonate solution. The mixture was further transferred into 100ml flask and made up with distilled water. The resulting mixture was filtered to obtain a clear colourless solution to be read on a spectrometer at 380nM.The concentration of saponins was calculated from a standard graph based on known concentration of saponins. Results obtained are shown in table 4.5.Trypsin Inhibitor DeterminationTrypsin inhibitor content was determined using the method of Kakade et al; (1974).Portion of (0, 1.0, 1.4, and 1.8ml) of the diluted cowpea suspension were pipetted into triplicate sets of test tubes and adjusted to 2ml with distilled water. 2ml of trypsin solution was added to each tube and then placed in a water bath at 37°C.5ml of Benzoyl-DL-arginine-P-nitroanilide (BADA) hydrochloride, and after 2min, the reaction was terminated by adding 1ml of 30% acetic acid. After thorough mixing, the contents were filtered (Whatman NO 54) and the absorbance of the filtrate was measured at 410nm against a reagent blank.Trypsin unit (TIU) was arbitrarily defined as an increase of 0.01 absorbance units at 410nm per 10ml of reaction mixture. Trypsin inhibitor activity was defined as the number of trypsin units Inhibitors (TIU). Results obtained are shown in table 4.5.Amino Acids Profile Analysis of the Cowpea flour SamplesAnalysis of Amino Acids ProfileThe samples were run for Amino Acids Profile and results obtained were shown in Table 4.6. The results were obtained by ninhydrin colorimetric method of analysis of Rosen (1957). The extract was suitably diluted to 1ml; of this was added 0.5ml cyanide acetate buffer and 0.5ml of 3% ninhydrin solution in methyl cellulose. The mixture was heated for 15minutes in 100°C water bath. Thereafter, 5ml isopropyl alcohol water mixture was added and shaken vigorously. After cooling, the colour was read in a colorimeter at 570nM. The concentration of amino acids was calculated from a standard graph based on known concentration of various amino acids. Results obtained are shown in Table 4.6.Essential Amino Acids Profile Analysis.Eight essential amino acids (valine, isoleucine, leucine, lysine, histidine, methionine and phenylalanine and threonine) were extracted from the results obtained for amino acids profile in Table 4.6. Hence, results obtained for essential amino acids profile are shown in Table 4.7.Essential Amino Acid Index Analysis.Eight essential amino acids (valine, leucine, isoleucine, lysine, histidine, methionine, phenyalanine and threonine) were obtained as compared with FAO reference protein and egg protein (g/16gN) as shown in Table 4.8.The essential amino acids index of the cowpea flour samples (as shown in Table 4.8) were calculated using the formula below:
Water Absorption Capacity10ml of distilled water was added to approximately 2g of flour sample in a centrifuge tube. The tube was agitated in a vertex mixer for 2 minutes. It was the centrifuged at 2000r.p.m for 10 minutes. The clear supernatant was decanted and measured to obtain volume of water absorbed. Water absorbed capacity was expressed as volume of water bound by 100g dried flour (ml/g) as shown in Table 4.2.Oil Absorption Capacity10ml of refined vegetable oil was added to approximately 2g of flour sample in a centrifuge tube. The tube was agitated in a vertex mixer for 2 minutes. It was then centrifuged at 2000rpm for 10 minutes. The volume of free oil was decanted and measured to obtain volume of oil absorbed. Oil absorption capacity was expressed as volume of oil bound by 100g dried flour (ml/g) as shown in Table 4.2.Pasting Properties Determination of the Cowpea flour Samples.Rapid Pasting Method Using the Newport Rapid Visco AnalyzerPasting characteristics were determined with a Rapid Visco Analyzer (RVA) (Model RVA 3D+, Newport Scientific Australia, (1998). First, 2.5 g of flour sample were weighed into a dried empty canister; then 25 ml of distilled water was dispensed into the canister containing the sample. The solution was thoroughly mixed and the canister was well fitted into the RVA as recommended. The slurry was heated from 50-95°C with a holding time of 2 min followed by cooling to 50°C with 2 min holding time. The rate of heating and cooling were at a constant rate of 11.25°C per min. Peak viscosity, trough, breakdown, final viscosity, set back, peak time and pasting temperature were read from the pasting profile with the aid of thermo cline for windows software connected to a computer (Newport Scientific, 1998). The viscosity was expressed in terms of Rapid Visco Units (RVU), which is equivalent to 10 centipoises. Results obtained are shown in Table 4.3.Analysis of MineralsAnalysis of some Minerals in the Cowpea flour SamplesThe samples were analyzed for some metals using standard method. These include Calcium, Magnesium, Potassium, Sodium and Phosphorous. The results obtained expressed in percentage for the first three and in parts per million (ppm) for the last two as shown in Table 4.4.Sodium and potassium were determined using a flame photometer (corning, UK model 403) using NaCl and KCl to prepare the standards. Phosphorus was determined colorimetrically using spectronic 20 (Gallenkamp, UK) as described by Pearson (1976) with KH2PO4 as the standard. Other metals were determined using atomic absorption spectrophotometer (Perkin-Elmer model 403, walk CT, USA).Concentration of each metal was calculated from a standard graph based on known concentration of the metal.Anti-nutritional Factors in the Cowpea flour SamplesThe samples were analyzed for some anti-nutritional factors such as Phytates, Saponin, Oxalate and Trypsin Inhibitor. Results obtained are shown in Table 4.5.Oxalate determinationTotal oxalate was determined according to Day and Underwood (1986) procedure. To 1gm of flour sample, 75ml of 15N H2SO4 was added. Solution was carefully stirred intermittently with magnetic stirrer for one hour and filtered using Whatman No 1 filter paper. 25ml of the filtrate was then collected and titrated against 0.1N KMnO4 solutions till a faint pink colour appeared that persists for 30 seconds.The concentration of oxalate was calculated from a standard graph based on known concentration of oxalate.Results obtained are shown in table 4.5.Phytate DeterminationPhytate was determined using Reddy and Love (1999) method. 4g of sample was soaked in 100ml of 2% HCl for 5hrs and filtered. To 25ml of the filtered, 5ml of 0.3% ammonium thiocynate solution was added. The mixture was then titrated with Iron (III) chloride solution until a brownish-yellow colour that persisted for 5minutes was obtained. The concentration of phytate was calculated from a standard graph based on known concentration of phytate.Results obtained are shown in table 4.5.Saponins DeterminationThe procedure of Brunner (1984) was used in the determination of saponins. 2g of flour sample was added to 100ml of isobutyl alcohol (octanol) and left on a UDY shaker for 5hours. The mixture was later filtered with No1 Whatman filter paper. Thefiltrate was transferred and was saturated with magnesium carbonate solution. The mixture was further transferred into 100ml flask and made up with distilled water. The resulting mixture was filtered to obtain a clear colourless solution to be read on a spectrometer at 380nM.The concentration of saponins was calculated from a standard graph based on known concentration of saponins. Results obtained are shown in table 4.5.Trypsin Inhibitor DeterminationTrypsin inhibitor content was determined using the method of Kakade et al; (1974).Portion of (0, 1.0, 1.4, and 1.8ml) of the diluted cowpea suspension were pipetted into triplicate sets of test tubes and adjusted to 2ml with distilled water. 2ml of trypsin solution was added to each tube and then placed in a water bath at 37°C.5ml of Benzoyl-DL-arginine-P-nitroanilide (BADA) hydrochloride, and after 2min, the reaction was terminated by adding 1ml of 30% acetic acid. After thorough mixing, the contents were filtered (Whatman NO 54) and the absorbance of the filtrate was measured at 410nm against a reagent blank.Trypsin unit (TIU) was arbitrarily defined as an increase of 0.01 absorbance units at 410nm per 10ml of reaction mixture. Trypsin inhibitor activity was defined as the number of trypsin units Inhibitors (TIU). Results obtained are shown in table 4.5.Amino Acids Profile Analysis of the Cowpea flour SamplesAnalysis of Amino Acids ProfileThe samples were run for Amino Acids Profile and results obtained were shown in Table 4.6. The results were obtained by ninhydrin colorimetric method of analysis of Rosen (1957). The extract was suitably diluted to 1ml; of this was added 0.5ml cyanide acetate buffer and 0.5ml of 3% ninhydrin solution in methyl cellulose. The mixture was heated for 15minutes in 100°C water bath. Thereafter, 5ml isopropyl alcohol water mixture was added and shaken vigorously. After cooling, the colour was read in a colorimeter at 570nM. The concentration of amino acids was calculated from a standard graph based on known concentration of various amino acids. Results obtained are shown in Table 4.6.Essential Amino Acids Profile Analysis.Eight essential amino acids (valine, isoleucine, leucine, lysine, histidine, methionine and phenylalanine and threonine) were extracted from the results obtained for amino acids profile in Table 4.6. Hence, results obtained for essential amino acids profile are shown in Table 4.7.Essential Amino Acid Index Analysis.Eight essential amino acids (valine, leucine, isoleucine, lysine, histidine, methionine, phenyalanine and threonine) were obtained as compared with FAO reference protein and egg protein (g/16gN) as shown in Table 4.8.The essential amino acids index of the cowpea flour samples (as shown in Table 4.8) were calculated using the formula below: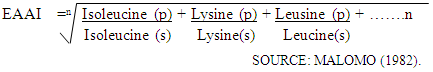 Where n is the number of essential amino acids.s is the essential amino acid of the standard protein which is egg protein.p is the value of the essential amino acid of the product.Statistical AnalysisData obtained were subjected to Analysis of Variance (ANOVA) and treatment means were separated using the New Duncan’s Multiple Range Test.The ANOVA was performed with Statistical Package for Social Sciences, SPSS 16.0 software (SPSS, 2007).
Where n is the number of essential amino acids.s is the essential amino acid of the standard protein which is egg protein.p is the value of the essential amino acid of the product.Statistical AnalysisData obtained were subjected to Analysis of Variance (ANOVA) and treatment means were separated using the New Duncan’s Multiple Range Test.The ANOVA was performed with Statistical Package for Social Sciences, SPSS 16.0 software (SPSS, 2007).4. Results and Discussions
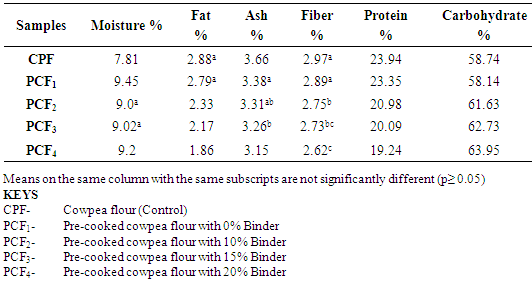
4.1. Effect of Treatments on Proximate Composition of Cowpea Flours
- The proximate composition of the control cowpea flour and the four pre-cooked cowpea flours with varying percentages of binder (corn-starch) are shown in Table 4.1.Values obtained in this study for the constituents of the control cowpea flour are in agreement with the values previously reported (McWatters, 1983; Ngoddy and Onuorah, 1986). Control sample recorded higher values for most parameters except carbohydrate. The result showed that the protein content of the cowpea flour decreased significantly from 23.94% to 19.24% while the carbohydrate increased from 58.74% to 63.95% with increasing addition of the cornstarch (Binder). This was due to the much higher carbohydrate content in the cornstarch than that of cowpea flour. Maize has been shown to contain about 72% starch (Nuss et al, 2010).The results also showed that the fat content of the cowpea flour decreased significantly from 2.88% in the control to 1.86% in the cowpea flour with 20% addition of cornstarch (binder). This was due to the fact that, though maize contains about 4% fat (Nuss et al, 2010), during processing of maize into cornstarch the fat portion is significantly removed. This explained the progressive decrease in the fat content of the cowpea flours with increasing addition of the cornstarch. Both the ash and fiber contents of the control cowpea flour and the pre-cooked cowpea flours showed no significant difference, ranging from 3.66% to 3.15% and 2.97% to 2.62% respectively. This was due to the fact that the treatments given did not affect these parameters (constituents) significantly.At the same time, these constituents are in the same range in both the cowpea and maize. Hence, the levels of addition of cornstarch to the cowpea flours showed no significant difference in the ash and fiber contents.
|
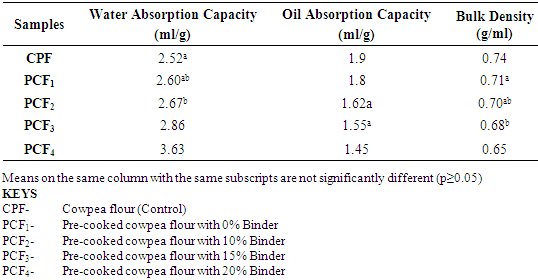
4.2. Effects of Treatments on the Functional Properties of the Cowpea Flours
- Water absorption capacity, which is the ability of flour to absorb water and subsequently swell, is a desirable functional property parameter in food systems to improve yield and consistency and give body to the food (Osundahunsi et al; 2003). The water absorption capacity was lowest (2.52ml/g) for the control (cowpea flour) and highest (3.63ml/g) for the pre-cooked cowpea flour with 20% binder. The water absorption capacity increased with increasing percentage of binder. Components of cowpea flour responsible for water absorption are protein, starch and cell wall materials (cellulose, pectin and hemicellulose) which form a matrix structure where capillary water is held contributing towards water absorption capacity of the flour (Kethiredipalli et al; 2002).The low water absorption capacity of cowpea flour may be due to weak association of amylose and amylopectin in the samples. Lorenz and Collin, (1990) and Malomo et al; (2012) both reported that water absorption capacity will be low if there is loose association between amylose and amylopectin in the native granules of starch and weak associative forces maintaining the granules structure.Increase in the binder percentage was responsible for the observed increase in water absorption capacity. Water absorption in flour correlates positively with the amylose content and also particle size of the cowpea flour (Adeyemi and Beckely 1986).The oil absorption capacity ranged between 1.90(ml/g) highest for the control (cowpea flour) and 1.45 (ml/g) lowest for the pre-cooked cowpea flour with 20% binder. Prinyawiwatkul et al (1997) reported that thermal treatment slightly increased the oil absorption capacity of cowpea flour, possibly due to increased surface hydrophobicity of protein which has been associated with the unfolding of protein when exposed to heat (and in this case during the drying operations). Increasing addition of the carbohydrate based binder correspondingly decreased the protein content, and subsequently a decrease in oil absorption capacity.
|
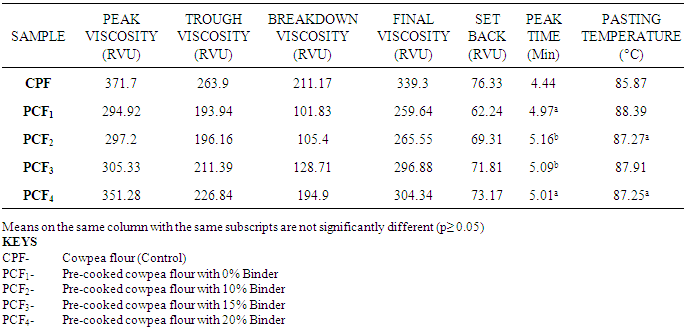
4.3. Effects of Treatments on the Pasting Properties of Cowpea Flours
- The Pasting characteristics indicated that the viscosities of the pre-cooked cowpea flour increased with the addition of cornstarch (used as a binder). The peak viscosities ranged between 294.92 RVU and 371.70 RVU (Rapid Visco Units). The results are in agreement with the Peak viscosity obtained for cowpea flour (340 RVU) according to Olapade et al. (2005) and various varieties of cowpea starches (890 to 127 RVU) as reported by Henshaw and Adebowale (2004).High peak viscosity is an indication of high starch content (Osungbaro, 1990) and it is also related to water binding capacity of starch (Adebowale et al; 2005). The low peak viscosities observed in pre-cooked samples are indicative of various degrees of starch gelatinization. The starch granules were completely gelatinized and therefore recorded low viscosities. However, these values increased with increasing levels of binder (cornstarch flour) in the cowpea flour. Low peak viscosity could be indicative that flour may not be suitable for products requiring high gel strength and elasticity, while being suitable in the preparation of complementary foods (Onimawo and Egbekun, 1998).Trough viscosity (holding strength or hot paste viscosity or ‘paste stability’) is the minimum viscosity value. This measures the ability of pastes to withstand breakdown during cooking (Olkku and Rha, 1978). Breakdown viscosity value is an index of the stability of starch (Fernandez and Berry, 1989). Final viscosity is the viscosity at the end of the test. It gives an indication of the ability of the cowpea flour to form a viscous paste or gel after cooking and cooling as well as the resistance of such paste to share-stress during stirring.Set-back viscosity involves re-association, retro-gradation or re-ordering of starch molecules. Low setback values in pre-cooked cowpea flour indicate that flour is not susceptible to retro-gradation. (aggregation of part of starch to form micro crystals which could precipitate). More so, pastes may be produced from the flour and stored with minimum retro-gradation (Oti and Akobundun, 2007). High setback (as in the control cowpea flour) viscosities are indicative of greater tendencies towards retro-gradation. High setback is also associated with syneresis or weeping. The setback viscosity of flours has been correlated with texture of various products (Adeyemi and Idowu, 1990; Michiyo et al; 2004).Peak time is the time at which the Peak viscosity occurred in minutes, and it is also indicative of the ease of cooking the product (Adebowale et al; 2008). Olapade et al; (2005) reported 72°C for cowpea flour and Elo faki et al; (1983) reported values of 65 - 73°C. Generally, treated (pre-cooked) samples had higher peak time than (the control) cowpea flour. This was in agreement with what Adegunwa et al; (2012) reported.Pasting temperature is the temperature where viscosities first increase by at least 2 RVU (Rapid Visco Units) over a 20sec period. It gives an indication of temperature required to cook the starch in the cowpeas beyond their gelatinization point (Olkku and Rha, 1978; Appelqvist and Debet, 1997).
|
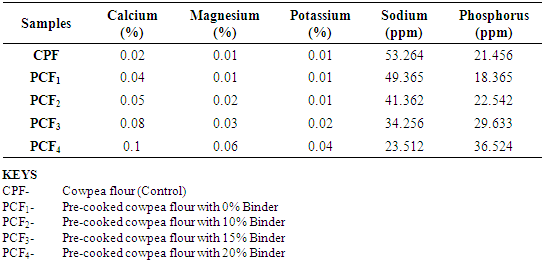
4.4. Effects of Treatments on Some Mineral Composition of the Cowpea Flours
- The combined effects of treatment and introduction of binder on the concentrations of minerals analyzed are shown in table 4.4. The sodium contents in the samples, compared to the control were observed to decrease because the binder (cornstarch) is low in sodium. The sodium contents ranged from 53.264 parts per million (ppm) in the pre-cooked cowpea flour with the addition of 20% cornstarch. On the other hand, the phosphorus contents were observed to increase significantly because the binder (cornstarch) is rich in phosphorus.The phosphorus contents ranged from 21.456 parts per million (ppm) in the pre-cooked cowpea flour with the addition of 20% cornstarch. Values of Calcium, Magnesium and Potassium ranged from (0.02% - 0.01%; 0.01% - 0.06%; and 0.01% - 0.04%) respectively.The values of these minerals, except sodium were observed to progressively increase with addition of increasing levels of binder.
|
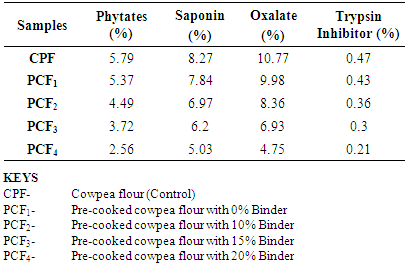
4.5. Effects of the Treatments on Anti-nutritional Factors in the Cowpea Flour
- Anti-nutritional factors affect protein digestibility (Ologhobo and Fetuga 1983, Abbey, 1976, Osagie, 1988). Most of them are destroyed by sufficient heat treatment (Leiner, 1979; Abbey, 1976) while some phenols (condensed tannis) that are fairly heat stable but located mainly in the seed coats can be significantly reduced by de-hulling (Phillip and Adams, 1983). In general, saponins in legumes are not destroyed during cooking (Osagie, 1988). Observed decrease here was due to the effect of the binder. Observed decrease in the concentrations of other anti-nutrients were due to the combined effects of de-hulling, heat treatment and addition of binder. Values obtained for phytates are in agreement with the range obtained by Afiukwa et al; (2011).The concentrations of phytates, saponins, oxalates and trypsin inhibitors in the control cowpea flour and the pre-cooked cowpea flours decreased significantly and ranged from 5.79% - 2.56%; 8.27% - 5.03%; 10.77% - 4.75% and 0.47% - 0.21% respectively. The anti-nutritional factors were observed to decrease progressively with increasing addition of cornstarch. The anti-nutritional factors were highest in the control cowpea flour and lowest in the pre-cooked cowpea flour with 20% cornstarch.On the other hand, anti-nutritional factors in cowpea have their beneficial aspects, for example, protease inhibitors are one of the most powerful cancer-protecting phytochemicals (Troll and Kennedy, 1983). Some phytates slow down the absorption of sugars and regulate insulin levels; beneficial in the treatment of diabetes and hyperlipidemia, high blood fat (Kakiuchi et al; 1986).
|
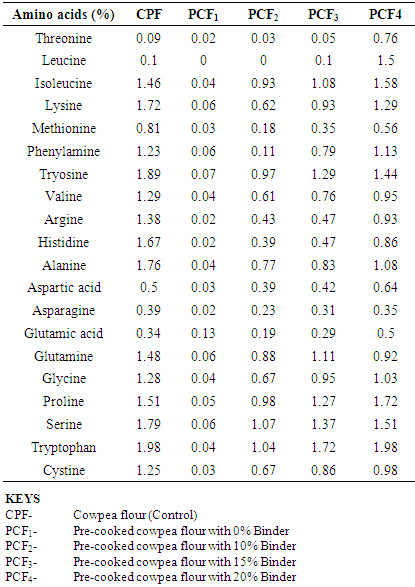
4.6. Effects of Treatments on the Results of the Amino Acids Profile
- From the amino acid profile in Table 4.6, the cowpea flour samples have a good quality of essential amino acids such as; leucine, lysine, phenylalanine, threonine and tryptophan; but deficient in sulphur containing amino acids. Hence the beneficial effects of maize as a binder, because maize is sufficient in the sulphur containing amino acids. Results showed agreement with Mosse and Pernollet, (1983).The results further showed a significant decrease in the amino acids profile between the control cowpea flour and the pre-cooked cowpea flours. This was due to loss of amino acids during processing.
|
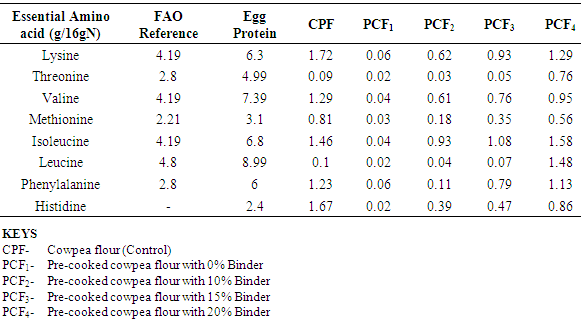
4.7. Effects of Treatments on the Essential Amino Acids Profile in the Cowpea Flours
- The essential amino acid in smallest supply in the food in relation to body needs is the limiting factor limiting amino acid because it limits the amount of protein the body can synthesize. Lysine is the first limiting amino acid in cereal grains whereas, that in legumes is methionine (Elegbede, 1988). The limiting amino acid may lead to poor utilization of amino acid by humans so that relatively more protein is required to meet the minimum requirement for protein synthesis.Leucine and threonine were the first limiting amino acids in the pre-cooked cowpea flour with 0% binder, while leucine was the first limiting amino acid in the pre-cooked cowpea flour with 15% binder (Table 4.7). The observed decrease in essential amino acid profile was due to loss of protein during processing. Lysine being the most vulnerable to stress of processing of all the amino acids, because of the position of the epsilon group could be easily knocked off during processing.
|
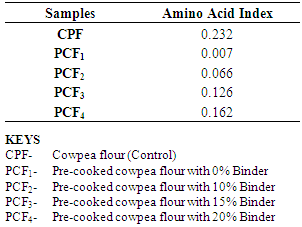
4.8. Effects of the Treatments on the Essential Amino acid Index of Cowpea Flours
- The observed decrease in essential amino acids Indexes were due to loss of protein during processing. Decrease in essential amino acids Indexes were due to decrease in values of essential amino acids from which these values were calculated.Essential amino acid index of control sample was recorded to be 0.232 and that of pre-cooked cowpea flours ranged from 0.007 to 0.162, with that of 20% binder having the highest value. The amino acids indexes for pre-cooked cowpea flours were approximately 55% and 70% for samples with 15% binder and 20% binder respectively when compared with that of control. The pre-cooked cowpea flour with 0% cornstarch had the lowest amino acid index (0.007) due to pronounced loss of protein during processing. However, amino acid indexes showed significant improvement with increasing addition of cornstarch. The amino acid index increased from 0.007 for pre-cooked cowpea flour with 0% cornstarch to 0.162 for pre-cooked cowpea flour with 20% cornstarch. This was due to the contributions made to the blend by the cornstarch. Cornstarch, from maize has been shown to be rich in sulphur-containing amino acids such as methionine, thus making it a good complementary food with cowpeas (Enwere and Ngoddy, 1986).
|
5. Conclusions
- A-short-cooking time cowpea flour for ‘moin-moin’ preparation was successfully produced. Nutritionally, though the product came out less than the control, the amino acids profile and the essential amino acids index suggested that its protein has moderate nutritive value. Convenience and time-saving are two-in-one unbeatable combination presented by this product. A product with such attributes would enhance domestic utilization of cowpea, hence increase in household levels and improved nutritional status.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML