-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(4): 70-74
doi:10.5923/j.food.20170704.02

Utilization of Sorghum (Feterita) Starch in Production of Fructose Syrup
Elamin A. Elkhalifa, Nadia K. A. Abdalla, Sarah, A. M. Abdelkareem
Department of Food Engineering and Technology, Faculty of Engineering and Technology, University of Gezira, Wad Medani, Sudan
Correspondence to: Elamin A. Elkhalifa, Department of Food Engineering and Technology, Faculty of Engineering and Technology, University of Gezira, Wad Medani, Sudan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

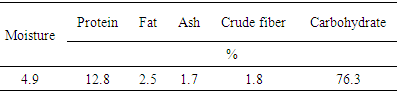
The experiments of this research were conducted for the isolation of starch from sorghum grains (Feterita) by wet milling process and subsequent use for production of fructose syrup by enzyme hydrolysis. Chemical composition and percentage of starch, amylose, water soluble amylose, total soluble sugars and reducing sugars were determined. Isolated starch was cooked by heating and liquefied by α–amylase and saccharified by amyloglucosidase. The glucose syrup produced was treated with isomerase to produce fructose syrup. The percentages of moisture, protein, fat, ash, crude fiber and total carbohydrate of Feterita were 4.9, 12.8, 2.5, 1.7, 1.8 and 76.3, respectively. Feterita grains contained 49% starch. Amylose and water soluble amylose were 32.2% and 5.6, respectively. Total soluble sugars were 2.9%. In hydrolysate reducing sugars, total soluble solids (TSS) and glucose contents increased successively during hydrolysis of starch. The glucose content was increased from 38.14 mmole/L to 360.35 mmole/L whereas glucose conversion to fructose reached 50%.
Keywords: Sorghum, Feterita, Starch, Hydrolysate, α-amylase, Amyloglucosidase, Isomerase
Cite this paper: Elamin A. Elkhalifa, Nadia K. A. Abdalla, Sarah, A. M. Abdelkareem, Utilization of Sorghum (Feterita) Starch in Production of Fructose Syrup, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 4, 2017, pp. 70-74. doi: 10.5923/j.food.20170704.02.
Article Outline
1. Introduction
- In the Sudan, starch is available from varieties of cheap sources (sorghum, maize, millet, cassava, sweet potato and potato) and the cheapest source is Feterita. The enzyme technology of glucoamylase is applied to the starch conversion process, giving added economic benefits to produce dextrose and dextrose syrups. In this process high starch concentrations can be used which requires less steam energy in subsequent evaporation steps. The conversion of glucose to fructose via-the action of enzyme glucose isomerase remains one of the most important transformations used in industry [1].The chemical isomerization of glucose to fructose is possible especially under condition of high temperature and alkaline pH [2-4]. However, chemical isomerization is not employed commercially because of the usual production of non-metabolizable materials such as psicose, formate and colored materials that are costly to remove [5]. The technique for the production of high fructose syrup (HFS) was first developed in Japan and later improved in United States [6]. In the U.S. corn starch is treated with the enzymes α–amylase and amyloglucosidease to produce glucose. The latter is then treated further with glucose isomerase to produce significant fructose content and hence greater sweetening capacity [5]. In the United States ten million tons of HFS are produced annually and used to replace sucrose in the majority of its uses [1]. Since 2000, production of high fructose corn syrup (HFCS) has declined by about 10 percent, with 2015 production totaling 8.5 million tons [7]. Fructose play an important role in the diet of the diabetics as it is only slowly absorbed by the stomach and intestinal tract and hence dose not influence the blood glucose level [8].In modern developments of starch industry, the starch can be converted easily to glucose and then further to HFS using immobilized enzymes process that can be recovered for reuse. Considering this, successful HFS industry can be developed in the Sudan adding economic value to the high production of sorghum crop in the country.The objectives of this study to utilize enzymes of α–amylase, amyloglucosidase and glucose isomerase to convert the Feterita starch to fructose syrup.
2. Materials and Methods
2.1. Materials
- Sorghum grains (Feterita) were purchased from Wad Medani local market. α–amylase, amyloglucosidase and isomerase enzymes were obtained from NOVO Nordisk A/S Denmark. The standard glucose kits were obtained from MDSS GmbH, Hannover, Germany.
2.2. Proximate Analyses
- The percentages of moisture, ash and fiber were determined using A.O.A.C. methods [9], while the protein content was determined by A.A.C.C. methods [10] and the fat content was determined by A.O.C.S. methods [11]. Total carbohydrates were obtained by difference between the sum of the other major compositions, namely moisture, protein, fat, ash and fiber from 100 percent.
2.3. Starch Isolation
- The starch isolated from Feterita grains by steeping first in an anti-bacterial mercuric chloride solution overnight and then by wet grinding in sodium chloride solution according to the procedure of Badenhuizen [12]. The isolated starch percentage was calculated using the following formula:
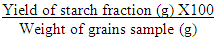
2.3.1. Amylose
- Amylose content in Feterita starch was released by treatment with diluted alkali according to the procedure of Williams, et al. [13]. The extracted amylose content was determined against amylose standard with iodine reagent at wavelength 600 nm.
2.3.2. Water-Soluble Amylose
- Water-soluble amylose content in Feterita starch using hot water was determined against amylose standard with iodine reagent at wavelength 600 nm according to the procedure of Juliano et al. [14].
2.4. Total Soluble Sugars
- Total soluble sugars content in defatted Feterita flour was extracted with aqueous ethyl alcohol, followed by treatment with phenol- sulphuric acid to produce golden yellow color. The absorbance was measured at 490 nm against glucose standard with different concentrations [15]. The percentage of total soluble sugars was calculated using the following formula:
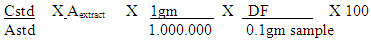 Where:Cstd = Conc. of standard (µg)Astd = Absorbance of standardAextract = Absorbance of 1ml sample extractDF = Dilution factor (100 ml)
Where:Cstd = Conc. of standard (µg)Astd = Absorbance of standardAextract = Absorbance of 1ml sample extractDF = Dilution factor (100 ml)2.5. Conversion of Sorghum Starch to Fructose
- Glucose was produced by enzymatic hydrolysis and then converted to fructose syrup, under optimum temperature, pH and incubation period required for the activity of the three enzymes as described by the method of Cheetham [16].
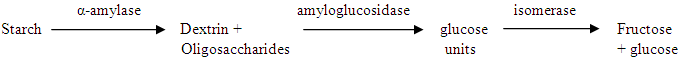
2.5.1. Determination of pH
- Electrometric method employing pH-meter with glass electrode (assembly) was used for pH measurements. The pH-meter was adjusted with standard buffer solutions. The pH of the hydrolysate after each enzyme treatment was recorded.
2.5.2. Determination of total Soluble Solids (TSS)
- The Abbe refractometer was adjusted at 20C to give zero reading using distilled water. 2-3 drops of starch slurry was transferred by a glass rod to the instrument. The reading was recorded in Brix to represent TSS [17]. The procedure was also used for determining TSS in hydrolysate sample.
2.5.3. Determination of Reducing Sugars
- Determination of reducing sugar using Lane and Eynon method. This method was used for determination of reducing sugars and other substances [18]. The percentage of reducing sugars was calculated using the following equation:
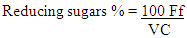 Where:Ff = the correction factor;V = the volume (ml) of the test solution used in the titration;C = the concentration (g/100 ml) of the sample in the test solution.
Where:Ff = the correction factor;V = the volume (ml) of the test solution used in the titration;C = the concentration (g/100 ml) of the sample in the test solution.2.5.4. Determination of Glucose Content
- The glucose content in starch mixture and hydrolsate sample was determined according to Tinder [19]. GOD-PAP enzymatic colorimetric method sold as a Kit was adopted for determination of glucose (MDSS GmbH, Hannover, Germany). The red Quinoneimine formed is proportional to the amount of glucose present in the sample as presented in the following reaction:
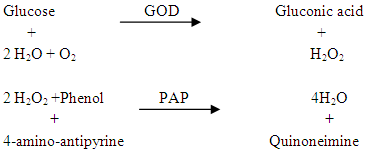 The amount of glucose was calculated as follows:
The amount of glucose was calculated as follows: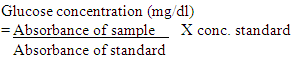
2.5.5. Determination of Fructose Content
- Fructose content was determined by amount of glucose which was converted to fructose by isomerase. Fructose content was calculated by subtracting the unconverted glucose in the enzymic reaction from the total glucose in the sample.
3. Results and Discussion
3.1. Chemical Composition of Sorghum (Feterita)
- The results obtained for the study of sorghum analysis are presented in Table 1.
|
3.2. The Isolated Starch
- The percentage of starch was found 49%. This result was higher than the result of Abd Elnour [23] who reported that the percentage of starch in Ferterita was found to be 44.2% in grains without decortication then increased to 57.05% in decorticated seeds once, and increased to 61.8% when Feterita was decorticated twice. Amylose and water soluble amylose content were found to be 32.2% and 5.6%, respectively.Buddair [24] reported that Feterita starch had the highest amylose content than other sorghum varieties (Dabar and Tetron). Rooney and Saldivar [25] reported that the amylose content in starch was 30%. This variation in result may be due to the genetic makeup of the sorghum varieties. The percentage of total soluble sugars was found 2.9% as reported in Table 2.
|
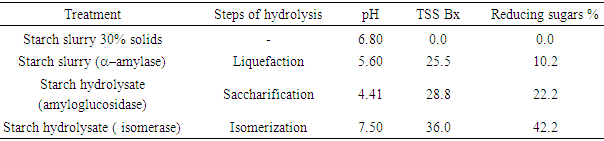
3.3. Starch Hydrolysate
- All polysaccharides can be hydrolyzed with acids or enzymes to yield monosaccharides. The results of pH, total soluble solids (TSS) and reducing sugars in starch slurry and hydrolysate samples are presented in Table 3.
|
|
4. Conclusions
- Feterita grain is relatively rich in protein and carbohydrates compared to other sorghum varieties. The starch isolated from Feterita grains had high amylose and water soluble amylose contents. Addition of isomerase enzyme converted the starch hydrolysate to a mixture of glucose and fructose whereas glucose conversion to fructose reached 50%. Feterita starch can be processed through enzymatic conversion to produce fructose syrup.Further research is needed to isolate the starch through dry milling process. Also further investigation is needed to study the physical and chemical properties of fructose syrup produced from Feterita starch.
ACKNOWLEDGEMENTS
- The authors express their sincere gratitude to Dr. Nagla Gasmelseed, in Nuclear Medicine Institute for her assistant and Mr. Hassan Ansari the head technicians in Food Analysis Laboratory.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML