| [1] | Alava V., R. and lim C., 1983. The quantitative dietary protein requirement of Penaeus monodon juveniles in a controlled environment, aquaculture; 30, 53. |
| [2] | AFNOR: 1995. Recherche et sélection de descripteurs pour l’élaboration d’un profile sensoriel, par approche multi dimensionnelle 1ère édition. Afnor. Paris; p: 1, 25. |
| [3] | AFNOR: 1994. Microbiologique alimentaire. Méthode de routine pour le dénombrement de Staphylocoque à coagulase positive par comptage des colonies à 57°C. NFV 08-057 AFNOR, p: 419-433. |
| [4] | AFNOR: 1994. Microbiologique alimentaire Directives générales pour les examens pour le dénombrement des Staphylococcus aureus Méthode par comptage des colonies. NFV 08-014, 150 6888, Afnor, p: 113-120. |
| [5] | AFNOR: 2001. Microbiologie des aliments. Méthode horizontale pour le dénombrement des Escherichia coli β- glucuronidase positive par comptage des colonies à 44°C, NF ISO 16649-2, Afnor: p: 1-8. |
| [6] | AFNOR; 1982. (Association Française de normalisation). Recueil des normes françaises des produits dérivés des fruits et légumes. In Jus de fruits. 1ère édition. Paris, France, p. 327. |
| [7] | Akpata, MI et OE Miachi 2001. Aspects nutritifs de deux plantes alimentaires: Une étude comparative préliminaire. Électronique J. Environ. Agric. Food Chem, 10: 2019-2025. |
| [8] | AOAC (Association of analytical chemists), 1970. Oficials methods of analysis, Association of analytical chemists, Washington, DC USA, USA. |
| [9] | Attama A. A., Adikwu M. U, 1999. The physicochemical properties of starch derived from Tacca involucrata. Nig. J. Nat Prod. And Med., 3, 71-73. Disponible sur le Web: <[ http://ajol.info/index.php/njnpm/article/view/11766]>. |
| [10] | Antia B.S., Akpan E.J., Okon P.A., Umoren I.U, 2006. Nutritive and Anti-Nutritive Evaluation of Sweet Potatoes (Ipomoea batatas) Leaves. Pakistan Journal of Nutrition, 5 (2), 166-168. |
| [11] | Biehl, RR et DH Baker 1997. Nutrition et diététique pour les soins de santé. 9 EDN, Churchill Livingstone, New York, pp: 223. |
| [12] | Bourely J. 1982. Observation sur le dosage de l’huile des grains de cotonnier. Cot. Fib. Trop, 27 (2), 183-196. |
| [13] | Busson F. 1971. Spirulina plantensis (Gni) Ceîtter et Spirulina geîtteri J de Toni, Cyanophycées alimentaires. Marseille: Armée français, service de santé, Parc Pharo. |
| [14] | Camille V. et coll. 2010. Comment évaluer la qualité gustative d’un produit; 6, 10. |
| [15] | Cheftel J-C., Cheftel H. 1977. Introduction à la biochimie et à la technologie des aliments. Volume 1. Technique et Documentation -Lavoisier, Paris, p. 383. |
| [16] | Cozzone A., Bursson F. 1970. Electrophorèse en gel de Polyacrylamide des protéines de S. plantesis et de S. gitleri. C.R.hebd. Séanc-Acad SC. Paris. |
| [17] | Dubois M., Gilles K.A., Hamilton J.K., Roben F. A.et al. 1956. Colorimetric method for determination of sugar and related substances. Anal. Chem, 28, 350-356. |
| [18] | Devani M.B., Shioshoo J.C., Suhagia B.N. 1989. Spectrophotometrical method for microdetermination of nitrogen in Kjeldahl digest. J. Ass. OFMF. Anal. Chem, 72 (6), 953-956. |
| [19] | Fenwick D.E., Oakenfull D. 1983. Saponin content of food plants and some prepared foods. J. Sci Food Agric, 34, 186-191. |
| [20] | Francis G., Kerem Z., Makkar H.P.S., Becker K. 2002. The biological action of saponins in animal systems: a review. British Journal of Nutrition, 88, 587–605.34. Guggenbühl N. Diététicien Nutritionniste. News 2003. Disponible sur le Web: <[http://www.healthandfood.be/html/fr/news/2003/2003-09-10.htm>]. |
| [21] | Fischer E. H., Stein E.A. 1961. DNS colorimetric determination of available carbohydrates in foods. Biochemical Preparation, 8, 30-37. |
| [22] | Garine I. 2002. Nourriture de brousse chez les Muzey et les Masa du Nord Cameroun. Méga-Tchad, CNRS, Paris, Proc. p. 13. |
| [23] | Guggolz R. M. J., Silviera V., Owens H. S. 1950. Determination of starch and amylose in vegetables. Application to peas. Anal. Chem, 22, 1156. |
| [24] | Goni I., Garcia-Diz L., Manas E., Saura-Calixto F. 1996. Analysis of resistant starch: a method for foods and food products. Food Chemistry, 56, 445–449. |
| [25] | Grases, F., BM Simonet, J. Perello, A. Costa-Bauza et RM Prieto 2004. Utilisation des phytates et nonphytate phosphore dans les poussins comme affecté par la source et la quantité de vitamine D3. J. Anim. Sei., 75: 2986-2993. |
| [26] | Gupta K., Barat G.K., Wagle D.S., Chawla H.K.L. 1989. Nutrient contents and antinutritional factors in conventional and non-conventional leafy vegetables. Food Chemistry, 31, 105-116. |
| [27] | Hercberg S. 1994. Fer, vitamines, oligo-éléments. I. Le fer. In Enseignement de la nutrition, tome 1, p. 121-131. |
| [28] | Kay D.E. (revised by Gooding E.G.B.) (b). 1987. Taro (Colocasia esculenta). In: Crop and Product Digest, No 2-Root Crops, Second Edition. London: Tropical Development and Research Institute, 32, pp. 308. |
| [29] | Koziol M.J. 1990. Afrosimetrie Estimation of Threshold Saponin Concentration for Bitterness in Quinoa. Journal of the Science of Food and Agriculture, 54 (2), 211-220. |
| [30] | Kunle, OO, YE Ibrahim et MO Emeje, S. Shaba et Y. Kunle. 1999. Effet du phytate sur l’élément biodisponibilité de la deuxième génération des rats. J. Trace Elem. Med. Biol, 17: 229-234. |
| [31] | Krauss Beatrice H., 1979. Native Plants Used as Medecine in Hawaï. Asia Pacific Journal of Clinical Nutrition, p: 64-74. |
| [32] | Lehninger A.L. 1982. La nutrition humaine. In Principe de biochimie. Edition Flammarion Médecine Sciences, pp. 753-789. |
| [33] | McDonald, J.H. 2009. Extraction, physico - chimiques et les propriétés de compactage de tacca amidon: Un excipient pharmaceutique potentiel. Starke, 55: 319-325. |
| [34] | Malassis L., Ghersi G. 1992. Evaluation nutritionnelle d’une situation alimentaire. In Initiation à l’économie agro-alimentaire. Ed. UREF, Hatier, p. 33-38. |
| [35] | Marigo G. 1973. Méthode de fractionnement et d’estimation des composés phénoliques chez les végétaux. Analysis, 2 (2), 106-110. |
| [36] | Manek R.V., Kunle O.O., Emeje M.O., Builders P. et al. 2005. Thermal and Sorption Profile of Starch Obtained from Tacca leontopetaloides. The University of Louisiana at Monroe, School of Pharmacy, 263 Sugar Hall, Monroe, LA 71209, USA- Starch/Sträke, 57 (2), 55-61. |
| [37] | Medoua N.G.J. 2005. Thèse de Doctorat/PhD. Potentiels nutritionnel et technologique des tubercules durcis de l’igname Dioscorea dumetorum (Kunth) pax: étude du durcissement post-récolte et des conditions de transformation des tubercules durcis en farine, p. 254. |
| [38] | Njintang Y.N., Mbofung C.M.F. 2006. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour, Elsevier, LWT, 39, 684-691. |
| [39] | Noonan S.C., Savage G.P. 1999. Oxalate content of food and its effect on humans. Asia Pacific Journal of Clinical Nutrition, 64-74. |
| [40] | Olesek W. et al. 2001. Steroidal saponins of Yucca schidigera Roezl. J. Agric. Food. Chem, 49(9), 4392-4396. |
| [41] | Parke D.V., Ioannides C. 1981. The role of nutrition in toxicology. Ann. Rev. Nutr, 1, 207-234. |
| [42] | Pingle, U. et BV Ramastin 1978. Analyse chimique des aliments. .. 7 EDN, Church Hill Livingstone, Londres, Royaume - Uni, pp: 72-73,138-143, 488-496. |
| [43] | Rouers B. 1996. L'eau, agent de détoxication alimentaire Étude de deux techniques de détoxication des plantes alimentaires utilisées par les Aborigènes Australiens. Altérité, 1(1). |
| [44] | RASAMOELINA H., 1999. La Spirulina de Madagascar: valeur nutritionnelle et qualité microbiologique. Mémoire de fin d’étude, Département I.A.A/E.S.S.A Antananarivo. |
| [45] | Sauvageot F. 1990. L’évaluation des denrées alimentaires aspect méthodologique. Lavoisier. Tee et doc. Paris, 195. |
| [46] | Sano Matohiko, 1999. Microbiologie de base de l’agriculture marine des crevettes. |
| [47] | Yisa, J., JN Egila et AO Darlinton 2010. Potentiels de matières premières du Nigeria marante polynesian sauvage (Tacca leontopetaloides) tubercules et d' amidon. J. Food Technol, 7: 135-138. |
| [48] | http://www.buzzle.com/articles/importance-of-carbohydrates.html Turner, BL, JM Paphazy, MP Haygarth et DI Mckelvie 2002. |



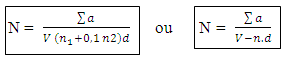 N: Numbers of colonies ∑a: The sum of the Ufc in two dilutions V: Volume of seeded inoculumn1: number of first dilution boxesd: dilution factor corresponds to the 1st dilutionn2: number of second dilution boxes n: number of box - sowing in surfaces for the Staphylococci coagulase (+) [2-4, 45]
N: Numbers of colonies ∑a: The sum of the Ufc in two dilutions V: Volume of seeded inoculumn1: number of first dilution boxesd: dilution factor corresponds to the 1st dilutionn2: number of second dilution boxes n: number of box - sowing in surfaces for the Staphylococci coagulase (+) [2-4, 45]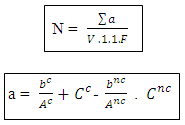 Ac: number of characteristic colonies planted out Anc: number of colonies non feature planted out bc: number of colonies of feature of presumed positive Staphylococci bnc: number colonies non feature of presumed positive Staphylococci Cc: number of characteristic colonies of positive Staphylococci presumed for it limps Cnc: does Sum of the columns of Staphylococci to positive coagulase identified in two limp. F: rate of dilution to the 1st dilution V: volume spread on every limps
Ac: number of characteristic colonies planted out Anc: number of colonies non feature planted out bc: number of colonies of feature of presumed positive Staphylococci bnc: number colonies non feature of presumed positive Staphylococci Cc: number of characteristic colonies of positive Staphylococci presumed for it limps Cnc: does Sum of the columns of Staphylococci to positive coagulase identified in two limp. F: rate of dilution to the 1st dilution V: volume spread on every limps 
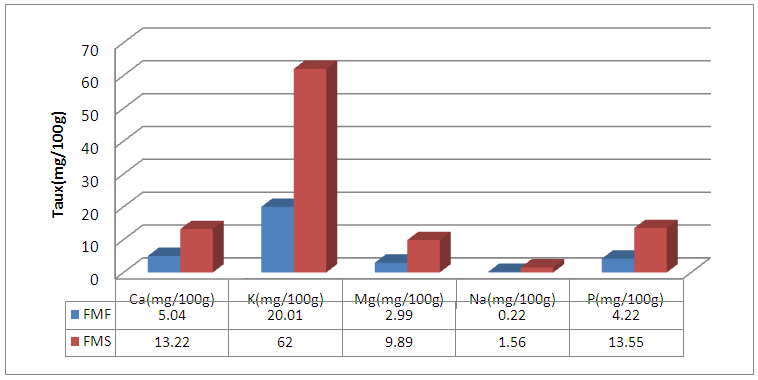
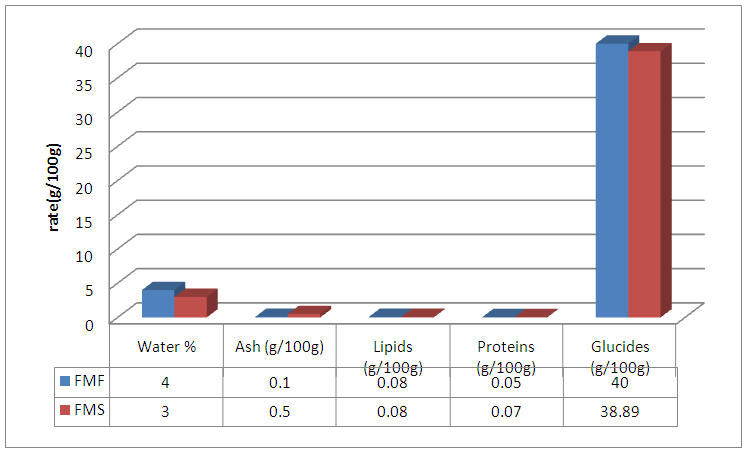
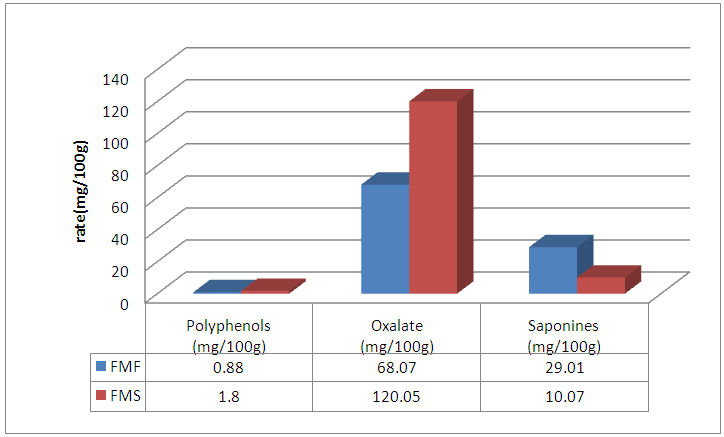
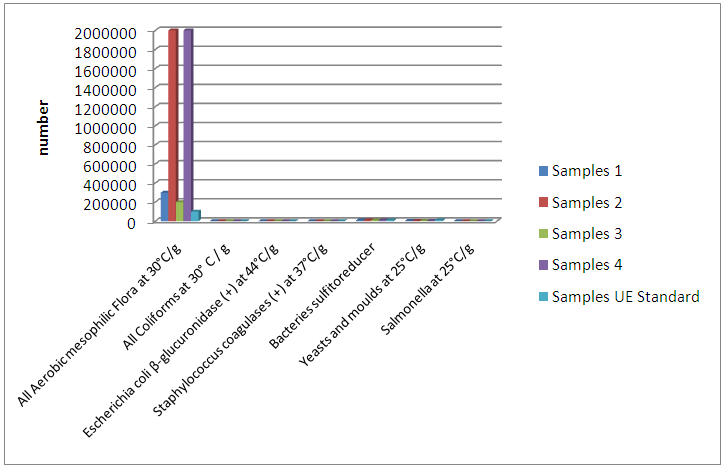
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML