-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(3): 43-50
doi:10.5923/j.food.20170703.01

Relationship of Average Titratable Acidity of Fermenters and Microbiological Stability of Fermented Food Products
Ngozi Nma Odu, Pascal Chinemerem Okoronkwo, Iheanyi Omezuruike Okonko
Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Iheanyi Omezuruike Okonko, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lactic acid bacteria are widely utilized as fermenters in the food fermentation industries. This study sought to ascertain the relationship between the average titratable acidity of fermenting LAB and the microbiological stability of fermented foods. Kunu-zaki, pap and yoghurt purchased from Choba market, Rivers State, Nigeria were cultured on Nutrient agar and de Man Rogosa and Sharpe (MRS) agar using spread plate technique. Isolates were identified using conventional methods and analytical profile index (API). The titratable acidity and pH reduction ability of LAB were determined using standard methods. Total viable count (TVC) and total lactic acid bacteria count (LABC) were as follows; kunu-zaki (TVC = 6.4 x 105 cfu/ml, LABC = TVC = 7.6 x 105 cfu/ml); Pap (TVC = 1.3 x 105 cfu/g, LABC = TVC = 6.8 x 105 cfu/g) and Yoghurt (TVC = 1.8 x 104 cfu/ml, LABC = TVC = 9.6 x 105 cfu/ml). Pediococcus acidilactii and Lactobacillus acidophilus were identified using API. The pH of LAB ranged from 3.7 to 4.5 while titratable acidity was 18.02 mg/ml lactic acid to 30.00 mg/ml lactic acid. Average titratable acidity was calculated as 22.3 mg/ml lactic acid (Kunu-zaki); 22.8 mg/ml lactic acid (Pap) and 25.9 mg/ml lactic acid (Yoghurt). The difference between LABC and TVC was 1.2 x 105 cfu/ml (Kunu-zaki); 5.5 x 105 cfu/g (Pap) and 9.4 x 105 cfu/ml (Yoghurt). It can therefore be deduced that the average titratable acidity of starter cultures gives an insight to the microbiological stability of the product. It is therefore recommended that for enhanced product quality, food processors should utililize a mixed culture with high average titratable acidity for fermentation.
Keywords: Fermenters, Microbiological stability, Average titratable acidity, Mixed culture, Lactic acid bacteria
Cite this paper: Ngozi Nma Odu, Pascal Chinemerem Okoronkwo, Iheanyi Omezuruike Okonko, Relationship of Average Titratable Acidity of Fermenters and Microbiological Stability of Fermented Food Products, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 3, 2017, pp. 43-50. doi: 10.5923/j.food.20170703.01.
Article Outline
1. Introduction
- Kunu (also known as kunu-zaki) is a non-alcoholic Nigerian fermented drink made from millet [1] (FAO, 1999). It is a popular drink widely consumed in the Northern Nigeria and prepared mostly by the Hausas [1]. However, kunu is now commonly used in southern Nigeria, due to its revitalizing potentials. The key ingredient used in preparing kunu-zaki is millet, very nutritious and beneficial to human health, thus making kunu a very nutritional drink [1]. Sorghum and maize are also used as a substitute for millet in preparation of kunu. Kunu is whitish when prepared with millet or maize, while the Sorghum variant is slightly brownish in colour [1]. Kunu is highly recommended for vegetarians and can be taken with bread or snacks [1].The traditional process of preparing kunu-zaki was described by Adeyemi and Umar [2]. This includes soaking millet grains, wet grinding with seasonings (pepper, ginger and cloves), wet filtering and fractional gelatinization of the slurry, as well as adding of sugar and bottling of product [1]. The fermentation briefly happens in the course of soaking for a period of 8 to 48 hr and this mainly involved yeasts and lactic acid bacteria (LAB) [1].Studies are currently ongoing to manufacture kunu-zaki with better-quality shelf-life [1]. However, Sopade and Kassum [3] in their study emphasized the implication of rheological features in structural analysis, processing, sensory evaluation and quality control of kunu-zaki. According to Sopade and Kassum [3], cumulative temperatures decreased viscosity of the product which did not change its rheological characteristics [1]. Adeyemi and Umar [2] in their study on storage of kunu-zaki showed that kunu-zaki had an approximately 24 hrs shelf-life, which was prolonged to eight days by pasteurization and stored in refrigerator [1]. Pap is one of the first weaning meals introduced to babies in Nigeria [1]. Pap is like our own version of the English custard [1]. Pap is quite popular and generally known in all parts of the country. Pap is popularly known as Akamu in Igbo, Ogi in Yoruba and Koko in Hausa and is made from wet yellow or white maize, guinea corn, millet and sorghum starch [1]. It has a characteristic bitter sense of taste that makes people desire it [1].This trio (corn, millet and guinea corn) combination makes pap a more nutritious meal for baby [1]. While corn is mainly a good source of carbohydrate, millet and guinea corn offers some proteins, vitamins and minerals that are very essential for baby’s growth and development [1]. Studies are presently ongoing in Africa to improve the pap processing with an objective of augmenting the nutritional worth, shelf-life and prospective therapeutic potentials [1]. In Nigeria, Olukoya et al. [4] reported the production of a particular pap with therapeutic features based on its capability to control diarrhea among infants. This discovery is of countless significance as pap is used as a widely acceptable weaning food for children in African countries [1].In another related study, Odunfa et al. [5] examined the likelihood of refining the restrictive lysine level in pap. Employing high lysine corn for refining the nutritional worth of pap was also reported by Banigo et al. [6] and Adeniji and Potter [7]. Drying up of water in pap by tray-drying or drum has been revealed to extend its shelf-life [1]. New studies have pursued to enhance the role of Lactobacillus species in the safety of pap [1]. Olasupo et al. [8] evaluated bacteriocin-producing Lactobacillus isolates to be active against common food-borne pathogens. In another similar study, bacteriocin also enhanced the shelf-life of pap, prolonging it by 10 days [9, 10].Yogurt, yoghurt, or yoghourt is a food made by bacterial fermentation of milk [1]. Yogurt cultures refers to bacteria employed in making yogurt. As one of the ancient and most common fermented foods, it is widely recognized round the world. Bacterial fermentation of lactose yields lactic acid, which acts on milk protein to offer yogurt its typical tang and texture. It is made by means of a culture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus bacteria. Additionally, other lactobacilli and bifidobacteria are also occasionally supplemented in the course of or subsequently culturing yogurt. Some countries need yogurt to comprise a definite quantity of bacteria colony-forming units; for example in China, smallest 1×106 CFU/g/ml is the prerequisite for the quantity of lactobacillus bacteria [11]. Yogurt is produce when milk is heated initially to around 185°F (85°C), to achieve milk proteins denaturation in order set altogether somewhat than produce curds. The milk is left to cool down to around 113°F (45°C) after heating [12]. At this point, the milk is mixed with bacterial culture and the 113°F (45°C) temperature upheld for 4-7hrs to permit fermentation.Titratable acidity refers to the hydrogen ion concentration of the total acidity in a solution determined by titrating with a strong base to a given endpoint [13, 14]. It plays a role in the intensity of sourness of an acid [15]. Titratable acidity as well as antagonistic activity of lactic acid bacteria (LAB) has been used by to determine the efficiency of the bacteria in fermentation. Lactic acid bacteria, the most widely used as starter culture in food fermentation [16], owe their desirable properties to their ability to produce organic acids (predominantly lactic acid) and other antimicrobial metabolites such as ethanol, hydrogen peroxide, diacetyl (an aroma compound) and bacteriocins [17]. They can be divided into two groups based on their products of hexose metabolism: homofermenters which produce lactic acid as the major or sole product of glucose metabolism heterofermenters which produce equimolar amounts of lactic acid, carbon (IV) oxide and ethanol from hexoses [18, 19]. LAB are generally regarded as save (GRAS) and offer many health benefits which include treatment of immunologic diseases such as allergic reactions, cancer, inflammatory bowel diseases (IBD) like chron’s disease and ulcerative colitis. Other health benefits of LAB include prevention of antibiotic associated complications, reduction of high blood pressure and cholesterol and management of lactose intolerance [19]. LAB have been widely used to ferment milk, vegetables, meat, fish and cereals. In addition, the heterofermenters play essential role in the production of alcoholic beverages [20, 21]. The study sets to evaluate the relationship between titratable acidity of fermenting organisms and the microbiological stability of fermented foods.
2. Material and Methods
2.1. Study Area
- Pap, yoghurt and kunu-zaki were purchased from Choba Market, Port Harcourt, Rivers State, Nigeria.
2.2. Media Used
- De Man Rogosa and Sharpe (MRS) agar (Lab M), MRS broth (Lab M), Nutrient agar (Titan Biotech), Triple Sugar Iron (TSI) agar (Titan Biotech), MRVP broth (Titan Biotech), Peptone broth (Titan Biotech), Simon’s Citrate agar (Titan Biotech), Christensen’s Urea agar base. Other reagents were of analytical standard.
2.3. Media Preparation and Sterilisation of Materials
- All the media used were prepared according to the manufacturer’s instruction. Glass wares were sterilized by autoclaving at 121°C, 15 psi for 15 min.
2.4. Isolation and Identification of Bacteria
- Lactic acid bacteria were isolated by modification of total lactic acid bacteria and total viable counts of Pap, yoghurt and kunu-zaki were determined by modification of the methods described by Mallesha et al. [22] and Ekwem [23] as follows; approximately 1 g of pap, 1 ml of yoghurt and 1 ml of kunu-zaki were each homogenized with 9 ml of sterile physiological saline to make the first dilutions. These were further diluted serially to 10-4 and plated out in duplicates on MRS agar and nutrient agar using the spread plate technique. After 48 h incubation at 37°C, the colonies were counted and only numbers ranging from 30 to 300 were used to calculate the colony forming unit. Isolates observed on MRS agar were then sub cultured on MRS agar while those observed on nutrient agar were purified on nutrient agar. After incubation at 37°C for 24 h, the isolates were identified based on their morphological and biochemical characteristics using the Bergey’s manual of determinative bacteriology [24]. Identity of selected LAB was confirmed by analytical profile index (API).
2.5. Identification of Selected Lab Using Analytical Profile Index (API)
- The selected LAB were identified by the method described by Agarry et al. [25]. The isolates were inoculated onto MRS agar and incubated anaerobically (Gas pak, BBL, Cockeyville, USA) for 24h. The isolates were sub cultured in 5ml MRS broth at 30°C for 12h. The cultures were then centrifuged at 9500g for 10min and the supernatant was decanted. The residue was further washed with sterile physiological saline to remove residual medium. A cell suspension was prepared according to the method described by McFarland [26]. Aliquots of the cell suspensions were transferred to API 50CH V3.0 strip wells (Biomerieux) which contained different sugars. The wells were covered with mineral oil and incubated at 30°C for 48h after which the strips were read for colour production indicating sugar fermentation. The colours were matched with the API 50CH V3.0 chart provided by the manufacturer. The identities of the isolates were then ascertained using API software.
2.6. Preparation of Cell Suspensions
- McFarland standard 1, equivalent to 3 x 108 CFU/ml, was prepared as prescribed by McFarland [26] in Pérez-Osorio et al. [27]. Approximately 0.1 ml of 1.0% BaCl2 was added to 9.9 ml of 1.0% H2SO4. This produced a precipitate of barium sulfate (BaSO4) causing the solution to become turbid. The absorbance of this solution was measured at 600 nm with the use of a spectrophotometer. This served as a standard to prepare cell suspensions of approximately 3 x 108 CFU/ml. Each LAB isolate was sub cultured in MRS broth at 37°C for 48 h following which it was centrifuged (Sorvall GLC – 4, Germany) at 400 rpm for 10 min and the supernatant fluid was discarded. The cells were washed with physiological saline to remove residual medium that will give turbidity that correlates falsely with cell numbers. The absorbance of the cell suspensions was also measured at 600 nm and very turbid suspensions were diluted further while more cells were added to less turbid suspensions until all were closely as turbid as the McFarland standard 1 preparation. The cell suspensions were cultured MRS agar using pour plate technique and the counts were corroborated with turbidity readings.
2.7. Titratable Acidity and pH Reduction Ability of Lactic Acid Bacteria
- The pH reducing ability and titratable acidity of LAB were determined by the methods described by Nwokoro and Chukwu [28] and Hwanhlem et al. [16].
2.8. Data Analysis
- Data generated from pH measurement, titratable acidity, TVC and LABC counts of the fermented food products were subjected to one-way analysis of variance with post hoc Turkey HSD using the statistical package for social sciences. Results were considered significantly different at p < 0.05.
3. Results
3.1. Total Viable Count (CFU/ml) and LAB counts (CFU/ml) of Kunu-Zaki, Pap and Yoghurt
- The TVC and LAB count of Kunu-zaki, Pap and Yoghurt are presented in Figure 1. It showed that Yoghurt had the highest number of LAB counts, followed by Kunu-zaki while the least LAB counts were found in Pap. Also, Kunu-zaki had the highest TVC, followed by Pap and Yoghurt showed the least TVC.
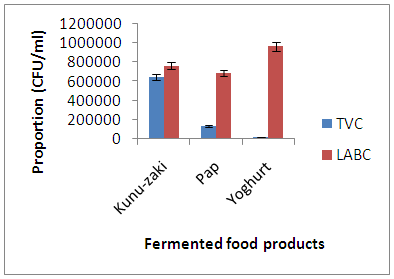 | Figure 1. TVC and LAB counts of kunu-zaki, pap and yoghurt |
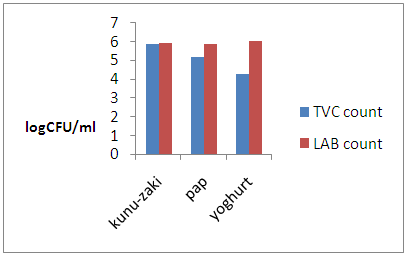 | Figure 2. Log CFU/ml counts of Kunu-zaki, Pap and Yoghurt |
3.2. Biochemical Characteristics
- The biochemical characteristics of isolates observed from Kunu-zaki, Pap and Yoghurt are presented in Tables 1 – 3. The organisms isolated from Kunu-zaki were identified as Pseudomonas sp., Corynebacterium sp., Pediococcus sp., and Streptococcus sp. (Table 1). Pseudomonas sp. and Corynebacterium sp. were most predominant, while Micrococcus sp., Pediococcus acidilactii and Streptococcus sp. were the least predominant (Table 1).Table 2 showed that organisms isolated from pap were identified as Corynebacterium sp., Aerococcus sp., Micrococcus sp., Pseudomonas sp., and Lactobacillus sp. Lactobacillus sp. were most predominant, followed by Corynebacterium sp. While Aerococcus sp., Micrococcus sp. and Pseudomonas sp. were the least predominant (Table 2).Table 3 showed that organisms isolated from yoghurt were identified as Micrococcus sp., Bacillus sp., Streptococcus sp., and Lactobacillus acidophilus. Micrococcus sp. were most predominant, followed by Bacillus sp. while Streptococcus sp. and Lactobacillus acidophilus were the least predominant (Table 3).
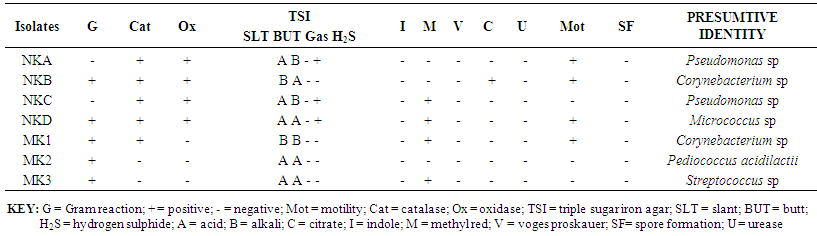 | Table 1. Biochemical characteristics of Kunu-zaki isolates |
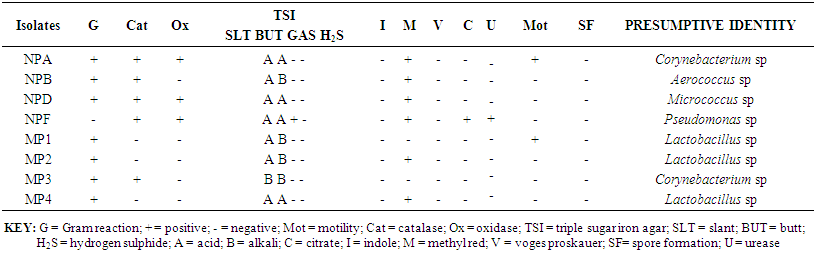 | Table 2. Biochemical characteristics of pap isolates |
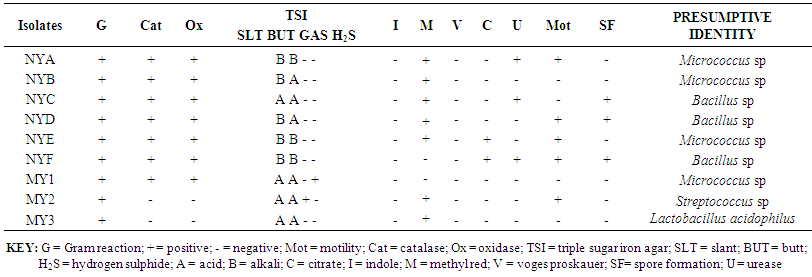 | Table 3. Biochemical characteristics of yoghurt isolates |
3.3. Analytical Profile Index (API)
- The API identification of selected LAB (MY3 and MK2) is presented in Table 4. It showed that the two selected LAB were identified as Lactobacillus acidophilus (MY3) and Pediococcus acidilactii (MK2).
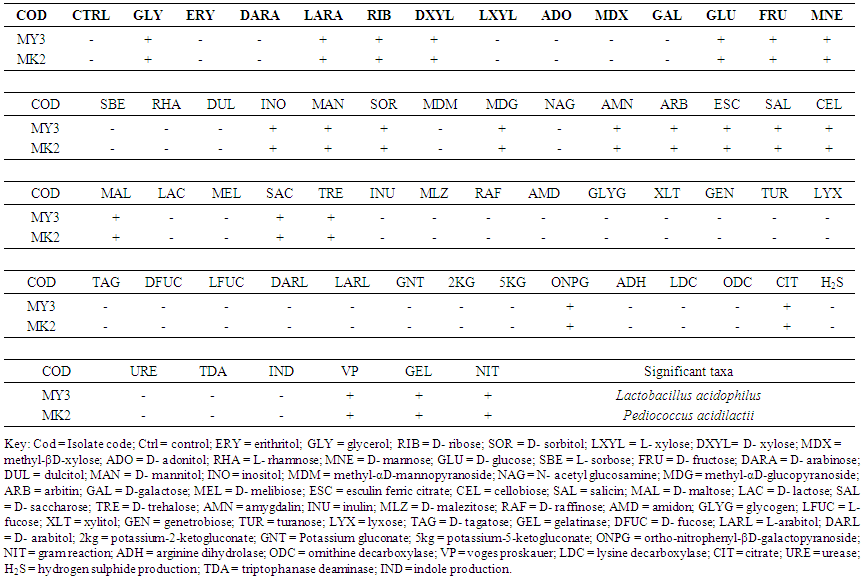 | Table 4. Analytical profile index (API) identification of selected LAB |
3.4. Titratable acidity and pH
- The titratable acidity and pH of LAB isolated from kunu-zaki, pap and yoghurt are presented in Table 5. It showed that MY3 had the highest titratable acidity of 3% lactic acid (30mg/ml) and lowest pH of 3.7. This was followed by isolate MP1 with Titratable acidity of 2.7% lactic acid (27.03mg/ml) and pH 4.0 and MK2 with Titratable acidity of 2.6% lactic acid (25.76mg/ml) and pH 4.0. They were therefore, the selected LABs. The other LABs had reduced the pH of the medium as follows: MK3, pH 4.4 with titratable acidity of 1.9% lactic acid (18.83mg/ml); MP2, pH 4.0 with titratable acidity of 2.3% lactic acid (23.40mg/ml); MP4, pH 4.5 with titratable acidity of 1.8% lactic acid (18.02mg/ml) and MY2, pH 4.1 with titratable acidity of 2.2% lactic acid (21.71mg/ml). However, the differences were not statistically associated (p>0.05).
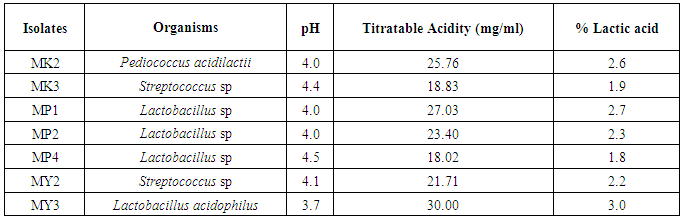 | Table 5. Titratable acidity and pH of lab isolated from kunu-zaki, pap and yoghurt |
3.5. Relationship between Average Titratable Acidity of Lab and Difference in Lab and Total Viable Counts
- The average titratable acidity of LAB and the difference between LAB count and total viable count of the kunu-zaki, pap and yoghurt are presented in Figure 3.
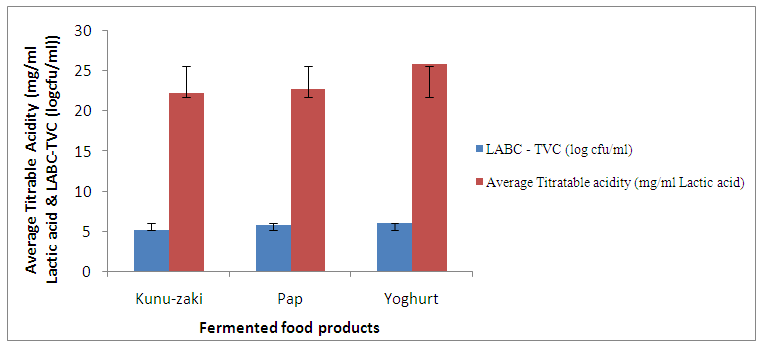 | Figure 3. Relationship between average titratable acidity of LAB and difference in LAB and total viable counts |
4. Discussion
- Pap, kunu-zaki and yoghurt are all LAB fermented products. The process of fermentation is inhibitory to spoilage microorganisms [16, 29]. However, fermented foods do not exhibit the same storage stability as was observed in kunu-zaki, pap and yoghurt analysed. Even though all the samples had higher LAB count than total viable count indicating good microbiological stability, yoghurt showed better stability than the other samples. Since unlike yoghurt, kunu-zaki and pap were purchased from local manufacturers, it can therefore, be deduced that the technological know-how of the manufacturer affects the microbiological stability of fermented foods.The products were observed to have higher LAB count than TVC count. This can be explained by the fastidious nature of the LABs which was observed during the initial isolation stage. Also, from the findings of Omenu and Adeosun [30], pH of ogi slurry decreased during fermentation and LABs were isolated from every stage of ogi production including the raw materials. This drop in pH is antagonistic to the growth of Gram negative spoilage and pathogenic bacteria. Hence, the difference in TVC and LAB counts. In this study, Kunu-zaki had a TVC count of log5.81CFU/ml and LAB count of log5.88CFU/ml. The TVC count of 6.4 x 105 cfu/ml from kunu-zaki conforms very closely to the findings of Aboh and Oladosu [31] who recorded a TVC of 6.0 x 105 cfu/ml from kunu-zaki sold in Garki, Abuja. Pediococcus acidilactii and Streptococcus sp isolated from kunu-zaki also agrees with the findings of Osuntogun and Aboaba [32] who isolated Lactobacillus and Streptococcus from kunu-zaki. Also in this study, Pap had a TVC count of log5.12CFU/ml and LAB count of log5.83CFU/ml Lactobacillus sp, Micrococcus sp and Corynebacterium sp isolated from pap is in accordance with the findings of Adegbehingbe [33] who isolated same organisms from fermenting sprouted and unsprouted maize slurry during ogi production.This study showed that yoghurt had TVC count of log4.26CFU/ml and LAB count of log5.98. The yoghurt brand tested had a TVC and LAB counts of 1.8 x 104CFU/ml to 9.6 x 105CFU/ml with the presence of Bacillus sp, Micrococcus sp, Streptococcus sp and Lactobacillus acidophilus. This partly conforms to the findings of Agu et al. [34] who isolated Bacillus sp, Streptococcus sp from yoghurt brands sold in Awka, Anambra State but recorded a total bacterial count (TBC) within the range of 3.2 x 105CFU/ml to 9.0 x 105CFU/ml. This difference was attributed to poor handling during processing and packaging. De et al. [35] isolated Bacillus sp, Streptococcus sp and Lactobacillus sp but recorded a TBC within the range of 0.3 x 104CFU/ml to 1.04 x 105CFU/ml from registered yoghurt brands and as high as 2.84 x 106CFU/ml from non-registered brands of yoghurt sold in Central market, Kaduna State. High bacterial counts from unregistered samples were also attributed to poor handling and lack of adherence to good manufacturing practice (GMP).The presence of Streptococcus sp and Lactobacillus acidophilus from the yoghurt brand tested confirms the use of these organisms as starter cultures as stated on the yoghurt label. However, Bacillus sp and Micrococcus sp are indicative of post pasteurization contamination as noted by De et al. [35] and Huck et al. [36]. Also, the presence of Bacillus sp supports the findings of Bramley and Mckinnon [37] that thermophiles such as Streptococcus thermophilus and Bacillus sp are able to survive raw milk pasteurization. However, the TVC and LAB count of the product used for this study implies that the yoghurt brand is of good sanitary quality.One mechanism by which lactic acid bacteria improve the keeping quality of food is by production of lactic acid which is antagonistic to spoilage and pathogenic organisms [16]. During glucose fermentation, homofermentative LAB produce lactic acid as the major product of glucose metabolism through the glycolytic pathway. The bacterial isolates from the samples tested were identified as LAB on the basis of being Gram positive, catalase negative, oxidase negative, non-sporulating and being able to grow on MRS agar. The LAB isolated from yoghurt had average titratable acidity (25.9 mg/ml lactic acid) higher than pap (22.8 mg/ml lactic acid) which is in turn higher than kunu-zaki (22.3 mg/ml lactic acid). When this is correlated with the difference between the LAB count and the total viable count, a similar trend is observed. This therefore implies that the average titratable acidity of the fermenting LAB affects the microbiological stability of the product.
5. Conclusions
- This study has shown that kunu-zaki and pap have high total bacterial count and were contaminated with Micrococcus sp, Bacillus sp and Pseudomonas sp. These organisms suggest poor handling during processing and packaging and can expose consumers to health hazards such as gastrointestinal infections. It can therefore be inferred that kunu-zaki and pap produced locally in Choba area of Rivers State, Nigeria are not of good sanitary quality. The yoghurt, however, recorded low bacterial counts. It is therefore of good sanitary quality. There was however no significant difference between the TVC and LAB count of the kunu-zaki, pap and yoghurt samples. Also from the study, it was observed that the microbiological stability of the fermented foods studied is dependent on the average titratable Acidity of the fermenter. It is therefore recommended that for enhanced product quality, food processors should utililize a mixed culture with high average titratable acidity for fermentation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML