-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(2): 32-37
doi:10.5923/j.food.20170702.02

Nutritional Evaluation of Roselle Seeds Oil and Production of Mayonnaise
Soheir M. El-Deab1, Heba E. Ghamry2
1Faculty of Education of Afif, Shaqra University, Saudi Arabia
2Faculty of Administrative & Home Economics College, King Khalid University, Saudi Arabia
Correspondence to: Soheir M. El-Deab, Faculty of Education of Afif, Shaqra University, Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Roselle seeds are waste that is left behind during processing of roselle for juices or other related products. Disposing of waste is highly undesirable both economically and environmentally. The aim of this study was to determine the chemical composition of roselle seeds and extracted oil from seeds, then study physico-chemical properties, fatty acid content and antioxidant component of oil. Finally, Use of roselle seed oil for production of mayonnaise for improving its oxidative stability. The physical properties of oil refractive index, specific gravity and color were tested the result were recorded 1.4643, 0.8985 and 0.067 at, respectively. Also the chemical properties which are peroxide value, free fatty acids, saponification value, and iodine value were tested the results were recorded 1.2, 2.5, 192 and 103, respectively. Total unsaturated and saturated fatty acids shows that the crude oil had 73.40% and 26.57% respectively. Major fatty acid found was Oleic acid (38.46%) followed by linoleic (33.25%), and palmitic (20.52%). Also, Total tocopherols were detected at an average concentration of 247 mg/100 g oil in roselle seed oil. Characteristics of roselle seed oil suggest that it could have important industrial applications. The data demonstrated that mayonnaise containing roselle seed oil (50%) was superior in sensory characteristics and oxidative stability as compared with control.
Keywords: Roselle seed oil, Mayonnaise, Oxidative stability, Fatty acid
Cite this paper: Soheir M. El-Deab, Heba E. Ghamry, Nutritional Evaluation of Roselle Seeds Oil and Production of Mayonnaise, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 2, 2017, pp. 32-37. doi: 10.5923/j.food.20170702.02.
Article Outline
1. Introduction
- Roselle (Hibiscus sabdariffa L.) is an annual botanical plant belonging to the Malvasia family cultivated in Egypt. There are more than 300 species of hibiscus around the world one of them is roselle (Hibiscus sabdariffa L.) which is member of the plant family malvaceae (Fasoyiro et al., 2005; Ismail et al., 2008). The calyces and leaves of the roselle are usually used for making jam, jelly, sauces and pickles, the petals of its flowers have been used in Egypt to prepare beverages, which have various important medical purposes Amin et al. 2008. Various studies have shown that roselle seeds contain much protein and oil (8-10) then…??. Roselle seeds contain about 14.9% protein, 21.2% crude fiber, 14.6% fats and oils, 35.6% carbohydrate. Roselle seed oil contains phytosterols and tocopherols [Nyam et al. 2009]. According to Nyam et al. (2009) roselle seeds contain approximately 15% on a dry weight basis (dwb) of highly unsaturated triacylglycerols and small amounts of other lipid components. The major unsaturated fatty acids found in roselle seed oil are oleic and linoleic acid. The presence of high linoleic acid shows that roselle seed oil could be a good source of essential fatty acids. Furthermore, roselle seed oil is a rich source of α-tocopherol. The α- tocopherol is the second major component in roselle seed oil. Tocopherols are well known as biological antioxidants that can prevent or retard the oxidation of body lipids, which include polyunsaturated fatty acids and lipid components of cells and organelle membranes. High levels of vitamin E detected in the oils, may contribute to greater stability in oxidation (Mohamed et al. 2007). In addition, the seeds also contain phytosterol compounds such as desmethylsterol (Nyam et al., 2009), which is known for its ability to reduce the absorption of dietary cholesterol when included in the human diet (Jones et al., 2000).Mayonnaise is a widely consumed food product (Cristina et al., 2005). Mayonnaise is a kind of semi-solid oil-in-water emulsion that prepared by emulsifying the oil with other components like egg, vinegar, and mustard. In multiphase systems, oxidative reactions are an interfacial phenomenon, which are affected by a wide number of different factors, such as the chemical composition and the physicochemical properties of the oil and water phases, the types of surfactants, and the surface area of the oil phase (Silvestre et al., 2000; Nuchi et al., 2002). Regarding oils, a wide variety of different types of oils have traditionally been used in food emulsions, including soybean, corn, canola, olive, safflower, and sunflower oils (Hui, 1992). The trend has been to replace traditional oils with more health-promoting oils, such as polyunsaturated lipids. Roselle seed oil is one of these beneficial oils.The objective of this study was to evaluated chemical composition of roselle seed. Also, extracted oil from seeds, then study physico-chemical properties, fatty acid content and antioxidant component of date roselle seed oil. Use of date roselle seed oil for industrial applications such as production of mayonnaise for improving its oxidative stability.
2. Materials and Methods
2.1. Materials
- Whole mature roselle (Hibiscus sabdariffa L.) seeds from Egyptian variety crop 2016 obtained from Aswan Government, Egypt. The seeds kept in a plastic container and stored in a deep freezer at −20°C until analysis.
2.2. Methods
- Oil extraction Five kgs of cleaning roselle seeds were ground and the oil was extracted by n-hexane (1:3 w/v) at room temperature. The miscella separated from the cake by filtration with Whatman filter paper. The filtrate miscella, were combined and n-hexane was removed under vacuum, at 50°C, by a rotary evaporator. The obtained oil was dried over anhydrous sodium sulphate then, directly analysed.Mayonnaise preparation Mayonnaise was formulated according to the following basic conditions: oil content (70%), egg yolk (18%), vinegar solution (8%), sugar (2%), and salt (2%). Four different formulations were prepared with 0% (control), 25, 50 and 75% of roselle seed oil RSO replacement of the total soybean oil content, respectively. The mayonnaise recipe was taken from Ma and Barbosa-Cánovas (1995). Sugar and salt were initially dissolved in vinegar solution. The mixture was then added to the egg yolk and homogenized by using Kitchen mixer for 1 min. Next, oil blend was added gradually at every 1 min interval for 9 times with 1 min rest at minute 4, 8, and 12. The blending was then continued for 3 min until the mayonnaise was homogenized. Three hundred grams of each mayonnaise sample were prepared for this study. The emulsion prepared was then incubated at 20°C in a plastic container for further analysis.
2.2.1. Chemical Analysis of Roselle Seed
- The moisture, ash and crude fiber contents were analysed according to standard methods described in AOAC (2000). Total Carbohydrates were determined by difference. All the proximate analyses were carried out in triplicate and the results expressed as percentage of the sample analyzed.
2.2.2. Physical and Chemical Properties of Roselle Seed Oils
- Refractive index, specific gravity, acidity, saponification value, iodine value, peroxidase value, and unsaponifiable matters were determined. Colormethod described by Lee et al., (2004) was applied. The absorbance of 5% (w/v) solutions of oil in chloroform was measured at λ = 420 nm using a "Spectronic 2000" spectrophotometer (Bauch & Lomb).
2.2.3. Fatty Acid Composition of Roselle Seed Oil
- The fatty acid methyl esters were prepared using benzene: methanol: concentrated sulfuric acid (10: 86: 4) and methylation was carried out for 1 h at 80-90°C according to Stahl (1967). The composition of fatty acid swas achieved by gas liquid chromatography analysis using a HP 6890 capillary gas chromatograph fitted with flame ionization detector, the column (60 m x 0.32 mm x 0.25 um). The column oven temperature was programmed at 10°C / min from 150 to 170°C, at 5°C / min from 170 to 192°C, holding five min then, from 192°C to 220°C during 10 min, holding 3 min and the nitrogen flow rate was 3 ml / min. Detector, injection temperatures, hydrogen and air –flow rates were 250°C, 230°C, 40 ml / min and 450 ml / min, respectively. The presented fatty acids were identified according to an authentic sample of fatty acids chromatograph under the same conditions.
2.2.4. Oxidative Stability of Roselle Seed Oil
- Oxidative stability was done using a Rancimat 679 apparatus (Metrohm AG, Herison, Swizerland) respecting these conditions: oil samples of 5.00 gm, temperature 100°C ± 2°C, and air flow rate of 20 L/hr (Mendez, et al., 1996).
2.2.5. Total Phenol of Roselle Seed Oil
- Total phenols in date pits oil were determined colorimetric at λ = 725 nm, with the Folin-Ciocalteau reagent, as previously done by Gutfinger (1981).
2.2.6. Determination of Tocopherols of Roselle Seed Oil
- Roselle seed oil (1.0 g) was saponified with 4 ml of 5% ethanolic pyrogallol (w/v), 1 ml KOH (100%) and boiled in a water bath for 3 min. Samples were then cooled, 30 ml distilled water was added and the mixture was extracted three times with diethyl ether. The combined extracts were washed with water to neutralise and remove fatty acid soaps. The extract was dried with anhydrous sodium sulphate and evaporated to dryness under a vacuum at 40°C. The residue was dissolved in 1.0 ml ethanol and 4.0 ml of benzene and dried under a stream of nitrogen. The residue was dissolved in 1.0 ml of ethanol and used for identification and quantification of tocopherol isomers by an HPLC method (Hatman and Kayden 1979) using a Agilent 1100 Series HPLC system equipped with a fluorescence detector and Phenominix C18, column (250 9 4.60 mm, 5 lm). The excitation wavelength used was 290 nm and the emission wavelength was 330 nm. An isocratic elution program was employed using a mobile phase containing methanol. The flow rate was 1.0 ml/min. The individual tocopherol homologues were calculated based on the calibration curve of standards of α, δ and γ tocopherols.
2.2.7. Sensory Evaluation of Mayonnaise
- Sensory evaluation was performed on mayonnaise samples produced from roselle seed oil. Prior to the sensory tests, the panellists (twenty persons) were trained to evaluate the attributes of the mayonnaise produced in this study and become proficient. The sensory evaluation of mayonnaise samples was conducted two times and the mean score values were reported in the text. The mayonnaise samples were rated on a 10 point scale (1, 2: bad; 3, 4: poor; 5, 6: fair; 7, 8: good; 9, 10 excellent). The mayonnaise samples, placed randomly on a codified plate with three-digit code, were served to each panellist. Judges were placed in different places to avoid communication during the evaluation and asked to score chips for taste, texture, appearance, color, odour and overall acceptability (Cochran and Cox, 1992).
2.2.8. Statistical Analysis
- Proximate analysis and crude oil properties were expressed as mean of three replicates ± standard deviation according to the method described by Steel et al. (1997).
3. Results and Discussion
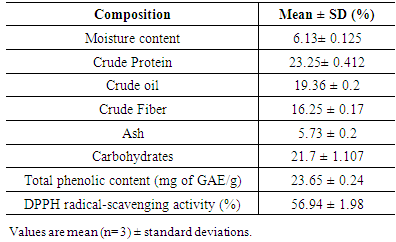
3.1. Chemical Composition of Roselle Seed
- The chemical composition of whole roselle seed is summarized in Table 1. Indicate that the roselle seeds contained low moisture content (6.13%), the low moisture content of seeds is usually relaxing the high stability and long shelf life of the seeds during storage, protection from mould growth and giving a high yield of dry weight. Roselle seed had relative high protein and fat contents (23.52% and 19.36%, respectively). The obtained protein content lower than the value obtained by Abu-Tarboush and Ahmed (1996). Meanwhile, the obtained fat content agree with the value (19%) reported by Mohamed et al., (2007). The relatively high fat and protein contents indicate that these seeds could become an excellent economic source for edible oil production.
|
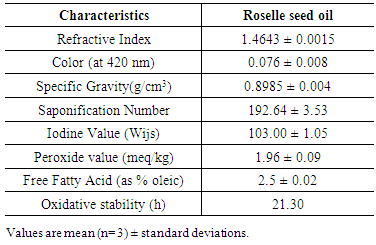
3.2. Physicochemical Characteristics of Roselle Seed Crude Oil
- The physicochemical characteristics of roselle seed crude oil are presented in Table 2. From the data, it could be observed that the oil had refractive index, 1.4674, specific gravity 0.8985 and bright yellow color (0.076 at 420 nm) the bright color of tested oil makes the roselle seed oil could be used directly without further bleaching processes in order to improve its color. The roselle seed oil was relatively low in iodine value (103.00). Iodine value indicates the degree of unsaturation while the saponification number (192.64) reflects the average molecular weight. The peroxide value (which is an indication of fat oxidation) was slightly high for crude oil (1.96), and this might be due to the conditions of storage and handling. Percentage of free fatty acid and oxidative stability in roselle seed oil was 2.10% and 21.30 hr, this may be due to the nature of roselle seeds, whereas many oil-bearing seeds such as olive, palm and rice bran contain high acidity oils Patterson 1989. The high oxidative stability of roselle seed oil could be at least partially attributed to its high tocopherol content, particularly γ -tocopherol. Also these results lower than the values obtained previously by Mohamed et al., (2007) found that the extracted roselle seed oil in a Soxhelt extractor using n-hexane at 65 to 70°C during the time necessary to quantitatively extract all the oil from the seeds had refractive index, 1.477; peroxide value, 8.63 and acidity, 2.24%. These differences may be attributed to the differences in the method of oil extraction.
|
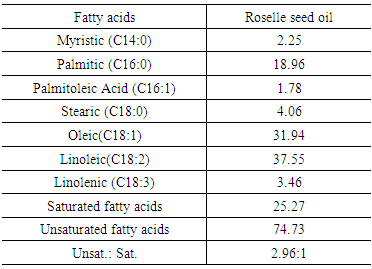
3.3. Fatty Acid Composition of Roselle Seed Oil
- Fatty acid composition of the roseelle seed oil shown in Table 3. The obtained data indicated that roselle seed oil consisted of 25.27% saturated and 74.73% unsaturated fatty acids. The percentage of total unsaturated fatty acids in roselle seed oil was about 78.2%, which was 2.96 times that of the saturated ones. Therefore, it could be concluded that roselle seed oil is a good source of mono- and polyunsaturated fatty acids which are effective in reducing both LDL and HDL cholesterols in serum. The most abundant fatty acids of roselle seed oil were linoleic (C18:2) and palmitic (C16:0), Stearic (C18:0) and Myristic (C14:0) which together composed about 92% of the total fatty acids. The major fatty acid found in roselle seed oil was linoleic acid (37.55%).
|
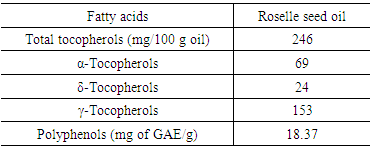
3.4. Tocopherol and Polyphenols of Roselle Seed Oil
- Total tocopherols were detected at an average concentration of 247 mg/100 g oil in roselle seed oil. This presents about 4 times the concentration of tocopherols in safflower, and 20 fold the tocopherols present in grape seed oil (Oomath et al., 2000). A qualitative estimation of tocopherols in roselle seed oil showed the presence of α, δ and γ -tocopherol (Table 4). All tocopherols, except β-tocopherol, were detected, γ -tocopherol being the most abundant species (62.19% total tocopherols), almost twice as much as α-tocopherol (28.04%), while δ-tocopherol was barely detected. Tocopherols are naturally occurring constituents found in vegetable oils in varying amounts. The presence of these compounds is important in relation to oil stability and nutritional labeling. Besides, consumption of these oils is recommended thanks to their beneficial effects on health (Gliszczynska-Swigło and Sikorska, 2004). The study of the phenols in vegetables is great interest, owing to the qualitative and quantitative differences appearing as a function of the species, cultivar and degree of ripening. Good amount of phenolic was estimated in roselle seed oil (Table 4), where in the level of total phenols, as determined by Folin–Ciocalteu method, was 18.37 mg/ g oil as gallic acid equivalents.
|
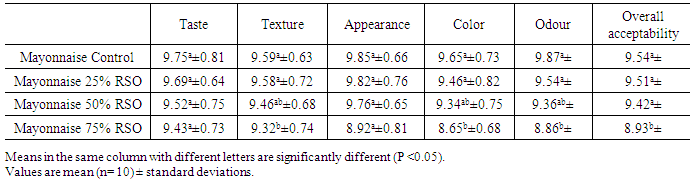
3.5. Sensory Evaluation of Mayonnaise
- A hedonic test was carried out among the four mayonnaise control and mayonnaise samples made by substitute corn oil by different levels from roselle seed oil RSO (0, 25, 50 and 75%) to evaluate the acceptability of the consumers in the sensory attributes of taste, texture, appearance, color, odour and overall acceptability. The results were tabulated in Table 5. The taste and appearance scores for the types of mayonnaise was not significantly affected by substitute of corn oil by RSO up to level 75%. These results are in accordance with (Hoffmann 1989 and Kishk 1997). Mayonnaise contained balanced proportions of salt, vinegar and spicing (mustard) that contributed to its taste. Because of the relatively high content of vinegar, a mayonnaise is characterized by a sour taste. Meanwhile, texture, color, odour and overall acceptability of control mayonnaise was have scores not significantly (P ≥ 0.05) than other samples mayonnaise up to 50% RSO in sensory characteristics. Color is one of the most important quality attributes of mayonnaise because color is the one criterion a consumer uses to select a mayonnaise brand from the grocer’s shelf. The yellowish color of mayonnaise is primarily provided by egg yolk carotenoids. The average data indicates that the received color scores of mayonnaise from 50% RSO had lower significant values between other samples. This indicated that 50% level of incorporation of roselle seed oil into corn oil was the optimum.
|
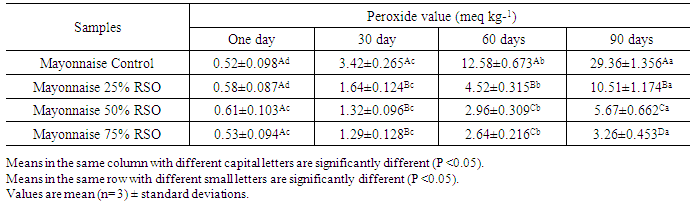
3.6. Effect of Storage on Peroxide Value of Mayonnaise
- Lipid oxidation is accelerated by reactions that take place at the surface of oil-in-water emulsion droplets (Mc Clements and Decker, 2000). Hydroperoxides were measured to determine the initial rate of oxidation because they are generally accepted as the first product formed by oxidation (Rossell, 1986). The peroxide values were significantly (P <0.05) increased in the prepared mayonnaise samples with the during storage period reaching to their highest values after 90 days. The substitution of corn oil by roselle seed oil at concentrations 50% and 75% could retarded the increase in peroxide value in prepared mayonnaise samples, and it reached its minimumvalues of 5.67 and 4.26 meq kg-1 oil, respectively, at the end of storage period (Table 6). Meanwhile, the peroxide value of control sample, arrived to highest value 29.36 meq kg-1 oil with significant difference (p < 0.05) compared to all the other prepared samples. High concentration of the roselle seed oil showed a great antioxidant activity. The antioxidant activity that appeared in the prepared mayonnaise was due to the phenolics content and tocophrol in RSO. The oxidative stability of prepared mayonnaise can be improved by the substitution of corn oil by roselle seed oil at concentrations 50% and 75%.
|
4. Conclusions
- The results presented in this article suggest that roselle (Hibiscus sabdariffa) could be used as a source of protein, vegetable oil, crude fiber, high relative percentages of unsaturated fatty acid, natural antioxidants including phytosterols and tocopherols, Regarding these specificities, the value and the newel of this by product is to use in some food industries such as mayonnaise. This work clarified that the oxidative stability of prepared mayonnaise can be improved by the substitution of corn oil by roselle seed oil at concentrations 50%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML