-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(1): 19-27
doi:10.5923/j.food.20170701.03

Microwave Assisted Extraction of Bioactive Compounds from Food: A Review
Aghdase Sadeghi1, Vahid Hakimzadeh1, Behnam Karimifar2
1Department of Food Science and Technology, Quchan Branch, Islamic Azad University, Quchan, Iran
2Department of Food Science and Technology, Tehran Branch, Islamic Azad University, Tehran, Iran
Correspondence to: Vahid Hakimzadeh, Department of Food Science and Technology, Quchan Branch, Islamic Azad University, Quchan, Iran.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The microwave-assisted extraction is also considered as a novel method for extracting soluble products into a fluid from a wide range of materials using microwave energy. Natural bioactive compounds are produced as secondary metabolites; include a broad diversity of structures and functionalities. Some of those compounds can be found in nature at high concentration such as polyphenols but others can only be found at very low levels, so that massive harvesting is needed to obtain sufficient amounts, and their structural diversity and complexity make chemical synthesis unprofitable. The inherent difficulties in screening and producing these compounds have led to the development of advanced technologies. The commonly used methods for their extraction are the conventional liquid–liquid or solid–liquid extraction and the advanced include pressurized-liquid extraction, subcritical and supercritical extractions, and microwave- and ultrasound-assisted extractions. These new technologies could provide in the next few years an innovative approach to increase the production of specific compounds for use as nutraceuticals or as ingredients in the design of functional foods. Advantages of microwave heating compared to conventional processing methods include energy-saving rapid heating rates and short processing times, deep penetration of the microwave energy (which allows heat to be generated efficiently without directly contacting the work-piece), instantaneous and precise electronic control, clean heating processes, and no generation of secondary waste. Microwave energy processes for heating, drying, and curing have been developed for numerous laboratory-scale investigations and, in some cases, have been commercialized. MAE is also recognized as a green technology because it reduces the use of organic solvent. This review is aimed to discuss this extraction technique along with their basic mechanism for extracting bioactive compounds from plant matrix.
Keywords: Microwave synthesis, Natural bioactive compounds, Extraction
Cite this paper: Aghdase Sadeghi, Vahid Hakimzadeh, Behnam Karimifar, Microwave Assisted Extraction of Bioactive Compounds from Food: A Review, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 1, 2017, pp. 19-27. doi: 10.5923/j.food.20170701.03.
Article Outline
1. Introduction
- The search for natural bioactive compounds (NBCs) with potential for the treatment and prevention of human diseases and to meet other needs is currently a key topic in many laboratories and industries. These compounds efficiently interact with proteins, DNA, and other biological molecules to produce a desired outcome, which could be exploited for designing natural products-derived therapeutic agents [1].Nowadays, there is a marked trend in the food industry toward the development and manufacture of functional products. This new class of food products has seen great success in the market due to the growing of consumer interest for “healthy” food [2, 3]. NBCs are often synthesized in small quantities in nature and are present as conjugates or mixtures in extracts which require labor-intensive and time-consuming purification procedures [4]. Besides, the structural diversity and complexity of these molecules make their chemical synthesis unprofitable [4]. NBCs have been studied directly in their different natural matrices such as tea, olive oil, exotic fruits, plants, algae, microalgae, bacteria, and fungi. In this regard, an increasing number of scientific studies have been performed on these substances and their sources.Recently, much attention has been directed to the recovery of bioactive compounds from different industrial food residues: leaves, peels, barriers, seeds, wood, culls, rinds, pits, pulp, press cakes, marc, malts, hops, hulls, husks, spent grain, carapace of crustaceans and shrimp, algae and other fish by products [5]. Traditional extraction techniques such as Soxhlet, solid– liquid extraction (SLE), or liquid liquid extraction (LLE) are characterized by high volumes of solvents and long extraction times. These techniques often produce low extraction yields of bioactive components and present low selectivity [6]. To overcome the limitations of these conventional extraction methods, non-conventional techniques, free of toxic solvents, have been introduced [7, 8]. In addition, extraction processes for the separation of compounds have been developed to obtain highly purified products, rendering them useful in a wide range of applications. These technologies could provide an innovative approach to increase the production of the desired compounds.Among them, microwave assisted extraction (MAE) has been accepted as a potential and powerful alternative for the extraction of bioactive compounds from food industrial residues. In addition, MAE technique possesses many advantages compared to other methods for the extraction of compounds such as bioactive compounds [9] saving in processing time and solvent, higher extraction rate, better products with lower cost, reduced energy consumption (up to 85-fold savings) and waste [10, 11]. In addition, MAE technique possesses many advantages compared to other methods for the extraction of compounds such as bioactive compounds [9] saving in processing time and solvent, higher extraction rate, better products with lower cost, reduced energy consumption (up to 85-fold savings) and waste generation [10, 11]. MAE is a method to extract soluble products into a fluid from a wide range of materials using microwave energy [12]. Interest in Microwave-Assisted Extraction (MAE) has increased significantly over the past 5-10 years in particular medicinal plant research, as a result of its inherent advantages as special heating mechanism, moderate capital cost and its good performance under atmospheric conditions [13, 14].Manabe et al. [15] used microwave energy to extract pectin from mandarin orange peels. They found that by using microwave energy, they could extract about 5% more pectin in 15 min than could be extracted by conventional methods in 60 min. More recently Wang et al. [16] optimized the operating conditions of pectin extraction assisted by microwave. They concluded that the application of microwaves in the extraction of pectin from dried apple pomace dramatically reduced the extraction time. The extracted pectin after purification could be added to different food products, both to enhance the nutritional value and improve food texture in jams, fillings, sweets, among others. Considering that tomato is one of the most consumed vegetable product, the amount of industrial waste is an huge quantity. Furthermore, the industrial tomato pomace is reach of for this reason El-Malah et al. [17] decided to compare ultrasound and microwave extraction in order to find the best extraction yield and antioxidant activity. They concluded that MAE was an optimum method to extract lycopene in a cheap and eco-friendly way. Peanut skins are another low-value by-product of peanut blanching and roasting operations [17]. They have been shown to contain significant levels of phenolic compounds with demonstrated antioxidant properties. Microwave hydro diffusion and gravity (MHG) is a recently developed technology with massive potential for variety of extractive applications, for instance, the extraction of essential oil has been executed from rosemary leaves [18, 19], Spearmint (Mentha spicata L.) and Pennyroyal (Mentha pulegiom L.) plant [20], and from citrus peel [18, 19]. The qualitative and quantitative studies of bioactive compounds from plant materials mostly rely on the selection of proper extraction method [21, 22].
2. Microwave Irradiation
- Microwaves (MW) are a form of non-ionizing electromagnetic energy at frequencies ranging from 300 MHz to 300 GHz. This energy is transmitted as waves, which can penetrate in biomaterials and interact with polar molecules into materials, such as water to generate heat [23]. Microwave energy acts directly on molecules by ionic conduction and dipole rotation and thus only polar materials can be heated based on their dielectric constant.Microwaves (MW) have the wavelengths between 1 mm and 1 m depending on the frequencies between 0.3 and 300 GHz. In electromagnetic radiation spectrum, the microwave radiation region is located between infrared radiation and radio waves [24]. In order to prevent any interference with cellular phone frequencies and telecommunications, the frequency of 2.45 GHz, which corresponds to a wavelength of 12.25 cm, is used for all domestic microwave ovens and chemical syntheses. The microwave heating is caused by the interaction of the electric field of radiation with the matter that can affect molecular actions, such as dipole rotations or ions migration. But, they cannot ionize the atoms crossed or change the molecular structure due to their low energy content [25]. Actually, it has a selective heating function. In other words, non-polar molecules are inert in the microwave electric fields while polar molecules with high dielectric constant and low molecular weight can selectively absorb microwave energy [26]. This selective heating of certain compounds may lead to the formation of microzones with a temperature much higher than the overall recorded temperature of the reaction bulk mixture. These high temperature microzones are called “hot spots” [27]. Thus a rapid enhancement of temperature is produced which may lead to an increase in the acceleration of chemical reaction rate [25]. For chemical reactions such as transestrification, that use microwaves as a means of heating, the polar molecules may bring more advantages [28].
3. Heating Mechanism
- Microwave heating (MWH) is a sub-category of dielectric heating at certain frequencies. MWH is a non-contact energy transfer process from electromagnetic energy into thermal energy, which indicates fast heating rate, if the electromagnetic energy is efficiently absorbed by the treated materials, under low microwave (MW) radiation. Conventional heat transfer methods, such as conduction, convection and radiation, need to overcome the heat transfer barrier and usually take a longer time to reach the desired temperature for the target materials in comparison to MW heating. The electromagnetic wave can be launched from an emitter and guided to the target. The mechanism of dielectric heating relies on dipolar and interfacial polarization effects. The electric field component of MW causes polar molecules (e.g. water) to rotate and try to align in both permanent and induced dipoles at certain frequency called dipolar polarization. Friction and collision generated by the increased molecular rotation and moving result in heat loss and MW heating [29]. It is believed that kinetic energy associated with vibrational, rotational and translational movement of valance electrons is responsible for the generation of thermal energy [30]. It was also reported that energy is dissipated in the form of heat called Max-well-Wagner polarization (interfacial polarization effects) [29, 31].In general, the higher the induced polarity, the greater the influence of MW, which indicates selective heating, uniform and volumetric heating characteristics of MWH. Based on the heating mechanism, MWH has the advantages of reducing the raw material pretreatment requirements, a substantial increase of product value, making it an efficient way of biomass energy usage [32].Dielectric lost tangent parameter (DLTP) quantifies a dielectric material's inherent dissipation of electromagnetic energy into heat, expressed by the ratio of dielectric loss factor (imaginary permittivity) to dielectric constant (relative real permittivity), where dielectric constant specifies the amount of electromagnetic incident energy reflected and absorbed by the treated material and the dielectric loss factor corresponds to the amount of electric energy dissipated in the form of heat within the material. DLTP is used to characterize the MW energy absorption and is helpful in studying the MWH processes. Most solid wastes show relatively low MW absorption when subjected to MW irradiation. These materials cannot be heated to the desired temperature owing to their inability to absorb sufficient amount of MW energy [33]. These problems can be solved by addition of MWAs into the heating system. The chemical structure, shape and size of microwave absorbers (MWAs) determine the number and intensity of the tiny micro-plasma (small or electric arcs) spots generated during MWH [30].
4. Principles and Applications of Microwave-Assisted Extraction
- Extraction is the first step of any medicinal or functional plant study, plays a significant and crucial role on the final result and outcome. Extraction methods are sometimes referred as ‘‘sample preparation techniques’’. The most common factors affecting extraction processes are matrix properties of the plant part, solvent, temperature, pressure and time [34]. Due to economics and environmental issues, food and chemical industries are facing the challenge of using new technologies in order to reduce energy consumption and CO2 emissions [35].The fundamentals of the microwave extraction (MAE) process are different from those of conventional methods (solid–liquid or simply extraction) because the extraction occurs as the result of changes in the cell structure caused by electromagnetic waves. In MAE, the process acceleration and high extraction yield may be the result of a synergistic combination of two transport phenomena: heat and mass gradients working in the same direction [36]. On the other hand, in conventional extractions the mass transfer occurs from inside to the outside, although the heat transfer occurs from the outside to the inside of the substrate (Fig. 1). In addition, although in conventional extraction the heat is transferred from the heating medium to the interior of the sample, in MAE the heat is dissipated volumetrically inside the irradiated medium. During the extraction process, the rate of recovery of the extract is not a linear function of time: the concentration of solute inside the solid varies, leading to a no stationary or unsteady condition. A series of phenomenological steps must occur during the period of interaction between the solid-containing particle and the solvent effectuating the separation, including (1) penetration of the solvent into the solid matrix; (2) solubilization and/or breakdown of components; (3) transport of the solute out of the solid matrix; (4) migration of the extracted solute from the external surface of the solid into the bulk solution; (5) movement of the extract with respect to the solid; and (6) separation and discharge of the extract and solid [37].
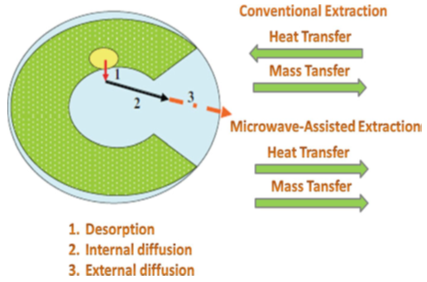 | Figure 1. Conventional extraction [37] |
5. Critical Analysis
5.1. Critical Analysis: Scenario of MAE of Botanicals
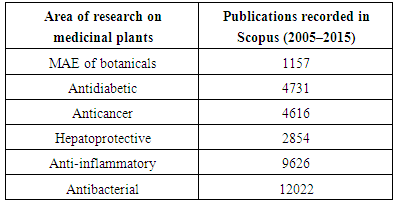
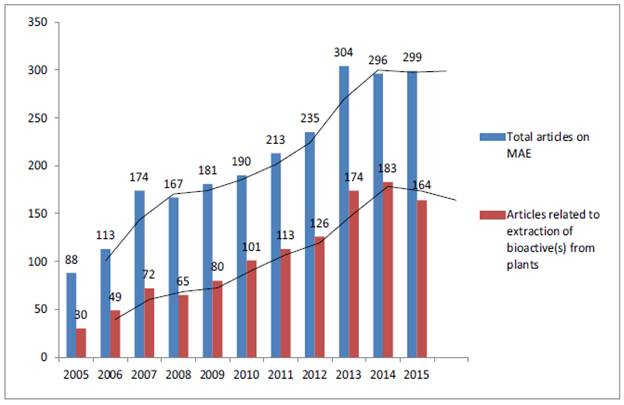
- Search results depicted in Fig. 2 clearly showed a trend that each year after 2010, minimum 50% of the publications that appeared on microwave assisted extraction where based on extraction of bioactive(s) from terrestrial plants. Pearson coefficient value of 0.982 clearly indicates a linear trend (2005–2015) between the total numbers of articles published in Scopus indexed journals on MAE and the number of articles actually related to extraction of bioactive(s) from botanicals. This is quite understandable from the fact that application of microwaves, pressurized liquids or ionic liquids are relatively new technologies which is being applied for the extraction of plant based bioactive(s). Huge technological advancements related to natural product research has taken place but innovation pertaining to extraction of botanicals has been on the slower side. This is evident from the fact that when it comes to drug discovery from natural products for the management of different diseases, publication rate of research papers has always been high. Publications recorded in Scopus database on MAE of botanicals was compared with the intensity of research on ethnopharmacology of medicinal plants where basically crude extracts are prepared and evaluated for their possible activity on different disease models (in vivo and in vitro) and the results are depicted in Table 1.
|
|
6. Conclusions
- As summarized, polar molecules under the microwave spectrum try to align themselves with the magnetic field lines, which are changed continuously. Thus the charged ion or molecular dipoles which interact with the changing magnetic field have to rotate rapidly. It results in the generation of energy in the form of heat due to molecular friction under MW irradiation. Transesterification process assisted by microwave technology is followed by a higher reaction temperature cites called hot spots. The review indicated that reaction in this situation is taken place in a temperature much higher than bulk temperature. Therefore, more efficient heating can be obtained compared to the usual conventional heating even more than sonochemical or supercritical reaction. This energy acts on the molecular scale [77]. From a technological point of view, recent batch microwave systems are smarter than earlier versions. These systems include libraries of predefined methods and can count the number of samples and recognize the vessel type loaded [78]. With this sequential approach, operators have the option to select an accurate temperature control for every sample or to handle different sample types with different extracting solvents, all within the same rack. In order to reach a batch-style approach, the main remaining hindrance is the sample-solvent separation after the extraction step. Most of the future fields of application are likely to focus on improving the flexibility of the latest introduced sequential systems. The capacity to control the extraction conditions for each sample should enable scientists to develop robust, reproducible methods for a diversity of matrices and chemicals. Overall, MAE appears to be an excellent alternative, as green extraction method, for the accurate determination of emerging organic pollutants, enabling its extension towards the regulatory environmental field.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML