-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2016; 6(5): 103-111
doi:10.5923/j.food.20160605.01

Effect of Starter Culture as a Source of Microbial Contamination on the Quality and Safety of Yogurt in Giza, Egypt
Mahmoud M. Motawee 1, Saleh M. Neveen 2
1Department of Nutritional Evaluation and Food Sciences, National Organization for Drug Control and Research, Giza, Egypt
2Department of Microbiology, National Organization for Drug Control and Research, Giza, Egypt
Correspondence to: Mahmoud M. Motawee , Department of Nutritional Evaluation and Food Sciences, National Organization for Drug Control and Research, Giza, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Yogurt is a fermented milk product, the popularity of yogurt can be attributed to its sensory characteristics and nutritional value. The microbiological characteristics of yogurt also contribute greatly to the quality and shelf life of the final product. The objective of this study was to evaluate the effect of starter culture as a source of contamination on the quality and the safety of Egyptian yogurt marketed in Giza, Egypt. Three groups of yogurt products were examined; 100 commercial yogurt samples were collected from local markets, Giza, Egypt, (Group I); 10 yogurt batches were manufactured using commercial standard starter culture (Group II); and 10 yogurt batches were manufactured using selected isolated cultures, (Group III). All groups were evaluated for the types of microbial groups, before and after storage period under refrigeration condition (7 ±1°C) for 14 days. The results revealed that, an undesirable change in commercial products as well as yogurt products made with commercial standard starter cultures during storage period. In contrast, group three, that using the selected isolated cultures, were prevented the presence of contaminants.
Keywords: Starter culture, Yogurt, Pathogenic bacteria, Safety
Cite this paper: Mahmoud M. Motawee , Saleh M. Neveen , Effect of Starter Culture as a Source of Microbial Contamination on the Quality and Safety of Yogurt in Giza, Egypt, International Journal of Food Science and Nutrition Engineering, Vol. 6 No. 5, 2016, pp. 103-111. doi: 10.5923/j.food.20160605.01.
Article Outline
1. Introduction
- Yogurt is one of the most popular fermented dairy product in Egypt. Moreover, yogurt fame came from its nutritional value and beneficial health effect [1]. The probiotics showed advantageous effects, including the production of antimicrobial compounds and used to prevent antibiotic-associated diarrhea [2, 3] which in turn led to increase consumers demand, especially children and other high-risk individuals.In the dairy industry, yogurt is produced from pasteurized milk fermented by the action of starter culture and generally, both Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus were used for yogurt making as a starter culture [4]. Recently, other strains have also been used as Streptococcus lactis, Streptococcus cremoris, Lactobacillus bulgaricus, Lactobacillus plantarum as well as Lactobacillus acidophilus that also exerts beneficial health effects and have been regarded as good sources of β-galactosidases, especially for functional food applications [5]. Moreover, β-galactosidases from -Streptococcus thermophillus, and Lactobacillus acidophilus are essential enzymes which hydrolyze lactose during commercial yogurt and cheese production. Streptococcus thermophillus β-galactosidase is active in the human digestive tract, improving digestion in lactose-intolerant individuals. There are many factors affecting the viability of the starter culture in yogurt such as pH, acidity, storage temperature, microbial contaminates and others [6]. In Addition, environmental stresses during dairy production and storage have some effects. Spoilage of dairy products was the main reason for the loss of yogurt effectiveness due to the losses of the viability of starter bacteria, that have been reported during storage, which in turn making the product unacceptable for human consumption [7, 8]. In the other hand, Yogurt is an excellent growth medium for a wide range of microorganisms, including fecal coliforms E. coli, Salmonella spp. And Pseudomonas spp., which unfortunately decrease the shelf-life of the product [9] which lower the grade and unsafe products. With spoilage development, some microorganisms produce metabolites as large amounts of extracellular hydrolytic enzymes lipolytic and proteolytic enzymes that hydrolyze milk fat and protein [10]; whereas others bacteria produce proteases and lipases (heat stable) that cause bitterness and off flavors in dairy products [11]. Undesirable microbes that can cause spoilage of dairy products include Gram-negative psychrotrophs, coliforms, lactic acid bacteria, yeasts, and molds. In addition, various bacteria of public health concern such as Salmonella spp., Listeria monocytogenes, Campylobacter jejuni, Yersinia enterocolitica, pathogenic strains of Escherichia coli and enterotoxigenic strains of Staphylococcus aureus may also be found in milk and dairy products. For this reason, increased emphasis should be placed on the microbiological examination of milk and dairy foods [12]. Therefore, the selection of good quality raw materials that have been used in yogurt manufacture, are essential in controlling microbial contaminations in food [10, 13]. In addition, temperature and duration of storage of the milk can cause its contamination [14, 15] also inadequate post fermentation hygiene prior to packaging or their ability to adapt different conditions such as transportation process and/or packaging, storage, transport and sales could increase chance of contamination [16]. MALDI–TOF MS (matrix –assisted laser desorption ionization time of flight mass spectroscopy) is a relatively novel technique in which a co-precipitate of a UV-light absorbing matrix and a biomolecule (such as pathogens) is irradiated by a nanosecond laser pulse. It is a very sensitive method, which allows the detection of low (1015 to 1018 mole) quantities of the sample with an accuracy of 0.1 - 0.01% [17]. MALDI-TOF MS could be used to obtain protein fingerprinting from whole bacterial cells, then by comparing these fingerprints to a reference database by the use of various algorithms for interpretation and comparison of these spectra [18]. Pathogen identification by this technique has the advantage of high confidence and speed (1 day for bacterial growth and analysis) together with negligible sample consumption (less than 1 pmol). With the use of MALDI-TOF MS, up to 100 bacterial isolates from milk of different dairy cows from several farms can be analyzed at once [19]. According to literature, knowledge, this is the first study that provides detailed data about the possible sources of contamination of yogurt in Egypt. This study offers the basis for further study to guarantee safe consumption of yogurt. Thus, the objective of this study was to determine the impact of different starter cultures used on the microbiological quality and safety of yogurt in Egypt during refrigerated storage.
2. Materials and Methods
2.1. Sources of Yogurt Cultures
- Three different sources of yogurt culture for making yogurt. (1) Commercial collected yogurt products from Giza governorate markets, Egypt. as a source of yogurt culture; (2) Commercial standard starter culture; (3) Isolated strains from the local yogurt for yogurt culture preparation.
2.1.1. Commercial Yogurt Collected Samples
- One hundred yogurt samples were randomly collected from local stores in Giza- governorate markets, Egypt, during the period extended from October 2013 to February 2014.
2.1.2. Commercial Standard Starter Culture
- A standard starter culture (YoFlex–YC-X11) which contained S. thermophilus and L. delbrueckii subsp. bulgaricus, were obtained from CHR HANSEN’s Lab., Inc. (Milwaukee), WI. This culture was obtained in Direct Vat Set (DVS) form and was stored at -18°C until use.For yogurt manufacture, activation of commercial standard starter cultures as the recommendations from the starter culture provider, a package of 250-g YoFlex–YC-X11 was thawed in a water bath at room temperature and then diluted 10-fold with sterile 10% dry milk.
2.1.3. Selected Yogurt Starter Culture (Stock Culture)
- The isolation of each collected yogurt strain from group I done on De Man, Rogosa and Sharpe (MRS) and M17 agar (Oxoid, UK) for enumeration of both Lactobacillus and Streptococcus, respectively as described previously [20]. The identification of each colony was done by Gram stain and phenotypic method [6, 21, 22]. One loop of each colony was then transferred into 5 ml of litmus milk then inoculated culture was incubated for 48 h at 37°C and stored in the refrigerator until use.
2.2. Confirmation of Strain Identity
- The identification of each yogurt strain was confirmed by MALDI-TOF MS using MicroFlex LT mass spectrometer (Bruker Daltonics) according to the manufacturer’s recommendations. Spectra were analyzed using the MALDI Biotyper automation control and the Bruker Biotyper 2.0 software and library (version 2.0, 3,740 entries; Bruker Daltonic GmbH Bremen Germany). Identification score criteria used were those recommended by the manufacturer: a score of ≥ 2.000 indicated species-level identification, a score of 1.700 to 1.999 indicated identification to the genus level, and a score of <1.700 was interpreted as no identification.
2.3. Detection of β-galactosidase Enzyme using SDS-gel Electrophoresis
- The β-galactosidase band was detected as described previously [23]. The isolated Lactobacillus and Streptococcus strains were cultured in MRS and M17 broth and then centrifuged at 10000 × g at 4°C for 10 min. after collection of supernatant, treated with 5% trichloroacetic acid (TCA) to precipitate the protein that secreted. Aaccording to [24], denatured samples were loaded into 12% continuous polyacrylamide gel preparation and run at 120 V for 4 h against a pre-stained protein molecular weight marker (Fermentas, USA). Gels were stained with Coomassie blue and de-stained with destaining overnight. Gels were digitally photographed then the intensity of bands were analyzed using Biogene software (France) and comparing with reference β-galactosidase enzyme.
2.4. Manufacture of Experimental Yogurt
- Pasteurized cow’s milk was purchased from a Giza governorate market (fat, 3.5%) and inoculated with starter culture. Two different groups of starter culture were used in the manufacturing of yogurt [25] as follow: Group II, (Processed yogurt-1 from commercial standard starter culture) obtained from CHR HANSEN’s Lab.; and Group III, (Processed yogurt-2 from selected isolated starter culture) that showed the highest β-galactosidase production. Ten yogurt batches from both group II and III were manufactured.Experimental milk was prepared by heating pasteurized cow’s milk at 85°C for 15 min and subsequently cooled to 42°C then it divided into two groups. Group II and group III were inoculated separately with 2% (W/V) starter. All groups were incubated at 42°C until the acidity was reached to 0.82-0.88 %, and incubation was stopped. Samples were immediately transferred to a refrigerator at 7 ±1°C (day 0) and stored for 14 days.
2.5. Microbiological Quality of Yogurt Samples
- For checking the quality of the collected and manufactured yogurt groups, the enumeration of microbial loads was done for the three groups of yogurt starter after and before 14-days under refrigerated conditions (7±1°C). Isolation of microbial loads was done on MRS, M17, and nutrient agar (Oxoid, UK) in duplicates then were incubated at 37°C for 24 h. as described earlier [15]. Growth was calculated as log10 colony forming a unit (CFU ml-1).
2.6. Chemical Analysis
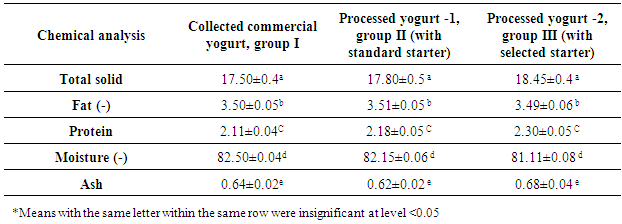
- Samples were analyzed for pH and titratable acidity before and after refrigerator condition. pH was analyzed using pH meter (Microprocessor pH meter, pH 537, WTW, Germany). Yogurt samples were also analyzed for moisture (method number 926.08), total solid (method number 4.1.03), fat (Gerber method), ash content, and total protein (method number 920.123) according to AOAC [26]. 2.6.1 Titratable acidity was detected using the method of AOAC [26]; method number 947.05 using phenolphthalein (Riediel DE HAEN AG, Germany) as an indicator. Yogurt samples were also analyzed for moisture (method number 926.08), total solid (method number 4.1.03), fat (Gerber method [27], ash content, and total protein (method number 920.123) according to AOAC [26, 28].
2.7. Sensory Evaluation
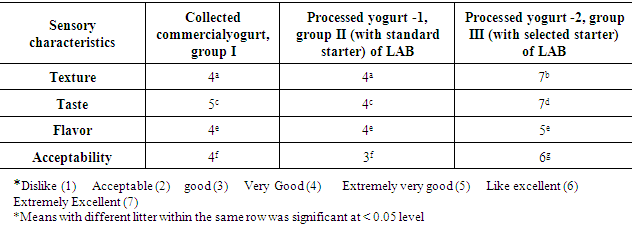
- Samples were subjected to sensory evaluation in order to check the texture, flavor, taste and overall acceptability of yogurt. After 14 days of storage period at 7°C ±1°C, consumer sensory panels were conducted at National organization for drug control and research, Food Science Department using panels of ten volunteers from the community. Commercial yogurts that purchased randomly from the local market and manufactured samples were tested. Each panel was presented with yogurt samples at room temperature (~22°C). Overall acceptability was based on flavor using a 7 -point Hedonic scale in which 7 = extremely excellent, 6 = excellent, 5 = Like excellent, 4 = very good, 3 = good, 2 = acceptable, 1 = dislike [29].
2.8. Statistical Analysis
- To analyze the total bacterial counts, before and after refrigerator storage condition among collected and manufactured samples, also chemical and sensory characteristics after storage were analyzed using one-way analysis of variance (ANOVA) was conducted using IBM SPSS statistics 20 (SPSS Inc., USA). All analyzes were done in triplicates. The generated data were subjected to analysis of variance (ANOVA) [30]. Differences among mean values were established with a mean values comparison using LSD’s test and were considered significant at p < 0.05.
3. Results and Discussion
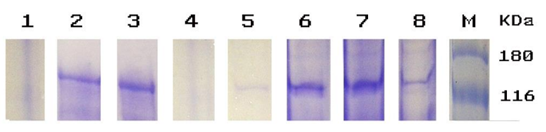
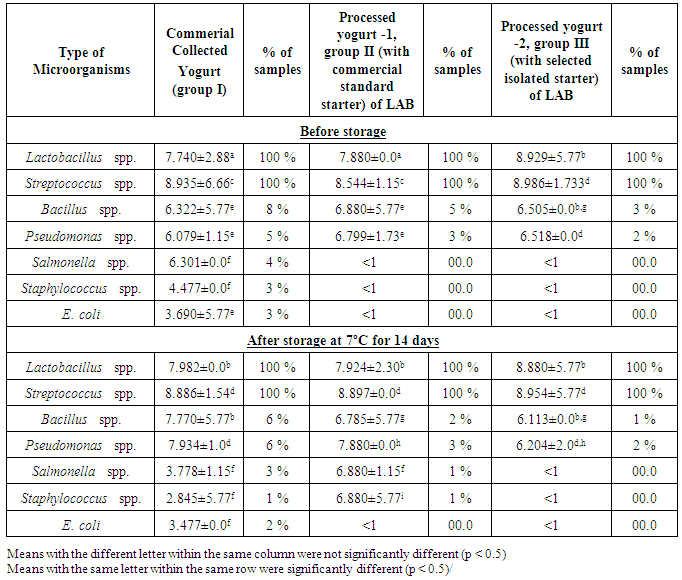
- The popularity of yogurt as a fermented dairy product recommended for both children and adults has led many microbiologists to focus on its quality and safety, both of which can be deteriorated due to microorganisms that can cause spoilage. Therefore, selection of lactic acid bacteria (LAB) which are safe for human use to provide a measurable defense against pathogens and are capable of producing high levels of β-galactosidase becomes paramount.Twenty yogurt culture strains Lactobacillus and Streptococcus were isolated from collected yogurt samples from the local markets, identified by phenotypic method and MALDI-TOF technique. All streptococci isolates were identified as S. thermophilus (90–99%); whereas Lactobacilli were identified as Lactobacillus delbrueckii, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus bulgaricus with excellent similar identification (97%) and for all other isolates from different yogurt groups using phenotypic tests correlated highly with MALDI-TOF/MS identification from 98-99% [31].The relationship of MALDI-TOF/MS with traditional method was supported [32, 33] who showed identical identification (99%) with the phenotypic method, indeed, MALDI-TOF/MS could be used as a replacement for the phenotypic and traditional method for LAB identification [31]. The molecular mass of β-galactosidase from twenty isolates was detected by SDS-PAGE (Figure 1). The results revealed that out of 20 strains, 6 strains (30%) showed highest β-galactosidase enzyme production and obviously seen with an expected molecular weight of 114 kDa. On the other hand, other two strains exhibited a weak protein band and could not be detected very clearly.The LAB strains with the high β-galactosidase enzyme as shown by SDS-PAGE were selected for the processing of yogurt as recommended in studies [2], [3]. Similarly, the production of β-galactosidase from S. thermophilus were reported [34]; whereas from L. bulgaricus reported that β-galactosidase production from yogurt isolate with a full 114 kDa band [23]. Moreover, [35] showed high production of the same enzyme from L. bulgaricus, L. lactis and L. casei with 116 kDa, that was in agreement with the current results.Based on β-galactosidase results, three strains Lactobacillus lactis, Lactobacillus bulgaricas, and Streptococcus thermophillus have been chosen for manufacture of group three yogurt. The viable microbial content of the collected yogurt and manufactured samples that were manufactured with commercial starter culture and selected isolated strains, were detected at zero time and after 14 days of storage under refrigerator storage conditions as presented in Table 1.A total of seven genera with variable existence was isolated from yogurt samples, namely: Staphylococcus, Bacillus, Pseudomonas, Salmonella, E. coli, Lactobacillus, and Streptococcus. The congruence between phenotypic tests and MALDI-TOF/MS technique for identification was between 96-99% of all isolates.The data showed that existence of lactic acid bacteria showed 100% in all (collected and manufactured) tested samples before and after storage under refrigerator condition and were maintained the highest counts compared to the other bacterial counts (Table 1). Although there was a variation in population counts, but still reflected the predominance of both bacteria in yogurt samples.
|
|
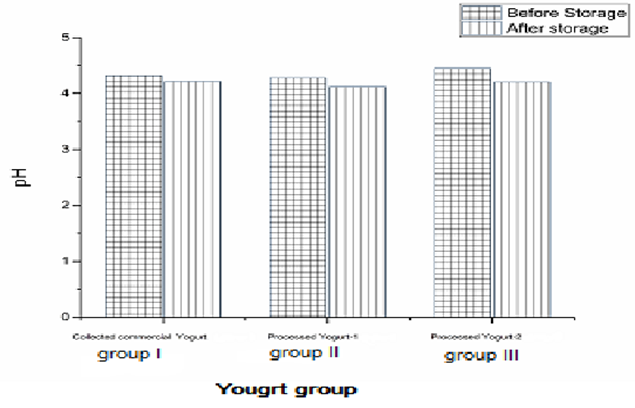 | Figure 2. Effect of three yogurt groups on pH after and before refrigerator storage condition |
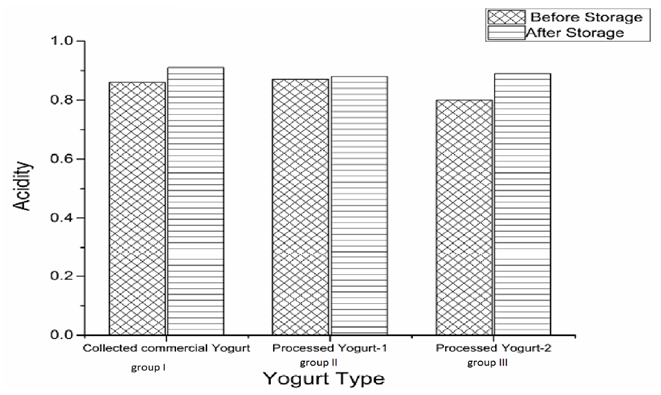 | Figure 3. Effect of three yogurt groups on acidity after and before refrigerator storage condition |
|
4. Conclusions
- This investigation has focused on the best selection of starter culture type that controls the quality and safety of yogurt from microbial contamination which has a strong connection to human health. The selection of quality of milk raw material also is very important. Among three tested yogurt groups, the selected starter culture (processed yogurt-2, group III) with high β-galactosidase content appeared to be a promising starter of high hygienic quality that has affected microbial contamination presence and improved the sensory characteristics even after refrigerator storage condition. Therefore, special attention should be given to starter culture used in yogurt manufacturing, which acts as an invisible potential reservoir of virulent pathogens that may constitute a public health hazard.This study represented an important evaluation (the first study in Egypt using MALDI–TOF/MS) of starter cultures in yogurt manufacturing in Egypt. It will reduce the gap between the research community and industry application. Successful application of the results of the study will protect the health of the Egyptian consumer as well as establish a novel method for the regulatory agencies for rapid identification of pathogens. Also, the present study has laid food foundation for further studies for starter culture type with highly β-galactosidase content in order to produce an efficient, safe and qualified product with concerning continuous HACCP monitoring for quality and safety of dairy products. In addition, MALDI-TOF/MS is considered as an alternative method for rapid microbial identification.
ACKNOWLEDGMENTS
- We appreciate Prof. Salam A. Ibrahim professor of food and Nutrition Sciences at North Carolina A&T State University, USA) for his kind contribution to this study by interpretation of results and the writing of manuscript. We also appreciate Dr. Islam M. El-Garawany, Researcher of biochemistry, Al-Monufeya University for his help in detection of β-galactosidase enzyme using SDS- gel electrophoresis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML