-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2016; 6(4): 81-86
doi:10.5923/j.food.20160604.02

The Effect of Malting Conditions on the Production of Non alcoholic Sorghum Malt Beverage
Ahmed G. M. Elgorashi1, Elamin A. Elkhalifa1, Abdel moneim E. Sulieman1, 2
1Department of Food Engineering and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
2Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
Correspondence to: Abdel moneim E. Sulieman, Department of Food Engineering and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study aimed to investigate the effect of malting conditions on malt quality to produce non-alcoholic sorghum malt beverage. Feterita sorghum cultivar was used to prepare the sorghum malt. Malting was carried out at two different temperatures of 25°C and 30°C for 4 and 5 days under air-rest and non-aerated conditions. Germination percentage was determined after germinating in an incubator at 30°C for 72 hours. The quality of sorghum malt in terms of malting losses, total loss, diastatic power and α and β amylase was examined. Malted grains germinated for 5 days at 30°C under non-aerated conditions had a greater effect on sorghum malt quality. For germination percentage, the results were 98% and 50% for maximum steep-out moisture. It is concluded that effective use of germination to process sorghum grain for beverage would require a control of the temperature and time. More research is needed in order to understand the adjustments made with different stages of processing of malted beverages.
Keywords: Sorghum, Diastatic power, Beverage, Iodine reaction, Germination percentage
Cite this paper: Ahmed G. M. Elgorashi, Elamin A. Elkhalifa, Abdel moneim E. Sulieman, The Effect of Malting Conditions on the Production of Non alcoholic Sorghum Malt Beverage, International Journal of Food Science and Nutrition Engineering, Vol. 6 No. 4, 2016, pp. 81-86. doi: 10.5923/j.food.20160604.02.
Article Outline
1. Introduction
- Sudan is considered the third country in Africa and seventh in the world of leading sorghum producer [1]. For millions of people in the Sudan sorghum is the most important staple food. Unfortunately, the most sorghum products in the Sudan are still traditional family art done in home in a crude manner and made under primitive conditions, which result in low yield and poor quality. However, sorghum is processed into a very wide of attractive and nutritious traditional foods. Sudan’s sorghum fermented foods are divided into two groups. One group encompasses the foods and beverages involving the use of germinated grain (malt), and the other group is composed of the foods and beverages prepared from only ungerminated grain [2].Sorghum malt is used to produce local beer namely merissa, an opaque beer and assaliya, a clear beer. These two products are to be found throughout the country and it is not known exactly what percentage of the sorghum consumed annually in the Sudan is turned into malt beverages, but all indications show that the proportion is very high.Merissa has great nutritional benefits because of the presence of the sprouted and fermented solids. Novellie and Schaepdrijver [3] wrote that sorghum beer was an excellent source of iron, manganese, magnesium and phosphorus. Budair [4] found that the sorghum cultivars, feterita, dabar and himeisis, contained fructose, glucose, raffinose and stachiose. Malting of the grain added maltose, isomaltose and maltotriose.Sorghum is increasingly the key ingredient in highly successful novel and non-traditional food and beverage products. In Nigeria, a wide variety of non-alcoholic malt beverages are very popular. These include both bottled “brewed” non-alcoholic malt drinks such as “Malta”. Sorghum malting in Nigeria has now become a major industry for malt beverage manufacture, with approximately 15,000 tons of sorghum being malted annually. Malt extract is a sweet, sugar-rich wort (unfermented beer), which can be concentrated by evaporation to a dark-coloured, richly flavoured syrup or dry powder. In Sudan sorghum malt is brewed as illegal business due to implementation of Islamic law since 1983 and public consumption are illicit. It is therefore wise to seek a way to prevent the consumption of harmful alcohol (about 6%) without depriving Sudanese of useful nutrients in sorghum malt beverages. The present study aimed to investigate the effect of malting conditions on production of non- alcoholic sorghum malt beverage.
2. Materials and Methods
2.1. Preparation of Samples
- Seeds of local sorghum (Sorghum bicolor (L) Moench) cultivar known as Feterita were procured from a retail outlet Wad medani market, Sudan. The grains were carefully cleaned and freed from broken seeds and extraneous matter. For the preparation of malt, the different steps were carried out according to the manual of laboratory procedures for quality evaluation of sorghum and pearl millet [5].Samples of grain (25 g), in nylon mesh bags (150 mm x 100 mm), were steeped in 0.2% NaOH at pre-determined temperature (25 and 30°C). After the first hour, the steeping vessels were drained and refilled with fresh tempered tap water. Grain at each pre-determined temperature was steeped for 24 hours, under one of two conditions of aeration, air-rest (AR) was achieved by draining steeping water from the grain, and it was given a 30 min air-rest six times within steeping period. Non-aerated (NA) was achieved by steeping the grain for the full steeping period in non-aerated water. At the end of steeping, the grains were centrifuged in spin dryer for one min at 300xg to remove the surface film of moisture and then the samples were weighed (steeped weight) [5] [6].The next step was the germination in which, the grains were spread evenly on trays lined with a single layer of moist filter paper and the trays were then covered loosely with cotton layer and stored at 25°C and 30°C for 96 and 120 h to allow germination to occur. The seeds were watered twice a day to prevent dehydration and turned daily to avoid root matting. At the end of the malting period green malt was weighed.Sorghum grains were dry-kilned at 50°C for 24 h in hot air oven (automatic electronic drying oven model OSK 6286). Dry malt was weighed. The malt was polished by manually rubbing samples with a dry muslin cloth and then polished malt was weighed. Then malted grains were ground in mill to pass through a 0.4 mm screen.
2.2. Malt Analysis
2.2.1. Germination Activity
- For determination of germination activity, seeds that developed roots and shoots were counted after 72 hr and the percentage was recorded. If, germination falls below 65% the grain is not viable enough to malt [5].
2.2.2. Steep-out Moisture
- The mass of the spin- dried, steeped grain was determined and the steep-out moisture calculated as percentage according to Gomez [5]. The result was expressed on a wet weight basis using the following formula:
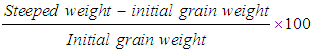
2.2.3. Malting Loss
- The loss of dry grain material as a consequence of the malting processes was calculated as percentage and expressed on a wet weight basis (Gomez, 1997) using the following formula:
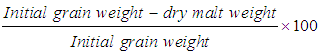
2.2.4. Total Loss
- Percentage of total loss was determined and expressed on a wet weight basis using the following formula:
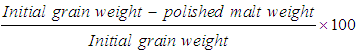 All the values were the mean of two determinations (Gomez, 1997).
All the values were the mean of two determinations (Gomez, 1997).2.2.5. Determination of Diastatic Activity
- Diastatic activity determination method was adapted from Daiber [7]. It was modified to a micro-method in order to reduce the sample size to 0.5 g malt. Ten grams of each polished malt sample were milled. 0.5 g of each milled malt sample was weighed into centrifuge tubes and 10 ml of peptone solution were added to each. The tubes were closed with Parafilm, shaken and stood in test- tube rack placed in the circulating water bath at 30°C. The samples were left in the water bath for 2.5 h to extract the diastatic enzymes and, during this extraction period, the tubes were shaken once every 20 min. At the end of the extraction, the suspensions were centrifuged for 2 min at about 3000 rev per min (1400 x g). Six 25-ml volumetric flasks were labeled as A1, A2, AB1, B1, B2, and BB1. 20 ml of buffered starch solution were pipetted into each flask and 4 ml 0.5N NaOH solution were pipetted into flask labeled AB1 and BB1 (this was the blank control). The timer was set for 30 min and, at 30-sec intervals, a 0.5-ml aliquot of the supernatant extract from centrifuge tube was dispensed into flasks. Immediately after extract was dispensed, each flask was closed by stopper, inverted twice, and placed in the water bath at 30°C. Exactly 30 min after the first extract was dispensed it was removed from the water bath and 4 ml of 0.5N NaOH were added to it. The other flasks were removed, again at 30-sec intervals, NaOH was added to the flasks to stop the digestion of the starch by the malt extract. This stage was carefully timed so that digestion was preceded for exactly 30 min in each sample flask although it was not critical for the blank. 50-ml Erlenmeyer flasks were labeled in the same way the 25-ml volumetric flasks were labeled, and 4 ml 0.05N alkaline ferric cyanide solution were pipetted into each Erlenmeyer flask. A 2-ml aliquot from each volumetric flask was transferred into respective Erlenmeyer flask. The Erlenmeyer flasks were placed onto the concentric boiling water bath and were left for 20 min. The flasks were allowed to cool before continuing. 10 ml acetic acid-salt solution and 0.4 ml potassium iodide solution were added to each flask and were titrated with 0.05N sodium thiosulphate with continuous stirring, until the blue color of starch iodine complex was disappeared.Diastatic power (DP) of malt was calculated as below, and expressed in diastatic units:
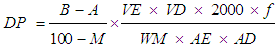 Where:A = titre of thiosulphate used for the sample (ml);B = titre of thiosulphate used for the blank (ml);AD = aliquot of digest for sugar determination (ml);AE= aliquot of extract for sugar determination (ml);f = normality of thiosulphate;M = % moisture content of malt;VD = volume of digest (ml);VE = volume of extract (ml);WM = weight of malt extracted (g).2000 ≡ dilution factor
Where:A = titre of thiosulphate used for the sample (ml);B = titre of thiosulphate used for the blank (ml);AD = aliquot of digest for sugar determination (ml);AE= aliquot of extract for sugar determination (ml);f = normality of thiosulphate;M = % moisture content of malt;VD = volume of digest (ml);VE = volume of extract (ml);WM = weight of malt extracted (g).2000 ≡ dilution factor 2.2.6. Determination of α and β Amylase
- α and β amylase determination procedure also was followed as the method of diastatic activity [5] with the following modifications:The centrifuge tubes Aα, Aβ, Bα and Bβ were labeled, and 0.5 g of sample A malt was weighed into two A tubes and 0.5 g of sample B malt into the two B tubes. In the two α tubes 0.316 g of calcium acetate was added, and in the β tubes 0.284 g of ammonium oxalate was added. Ten milliliters of peptone solution were added to each tube and it was extracted as before. After the samples were centrifuged, the same procedures for digestion and titration as in the method of diastatic activity were followed. Volumetric and Erlenmeyer flasks were labeled as follows: Aα1, Aα2, AαB1, Aβ1, Aβ2, AβB1, Bα1, Bα2, BαB1, Bβ1, Bβ2, and BβB1.
3. Results and Discussion
3.1. Germination Count
- Germination count of 100 seeds was made after 72 hours. The result was 98% that indicating the grain was viable enough to be malted. Studies on malting of sorghum showed that a germinative energy at 99% level can be achieved with some sorghum varieties. Sorghum with germinative energy greater than 95% is considered to be of good germination ability [8] [9] investigated malting properties of 16 sorghum varieties, they found that red feterita germinated at 28°C, 95% relative humidity and counted after 72 hours had 97% germination energy. Germination of grains is an essential part of the malting process because germinated, or poor germinated do not contribute to the enzyme development of the malt and uneven modification of the malt occurs. Sufficient enzyme modification of the endosperm substrates will not be achieved and will result in sub-optimal extract development.
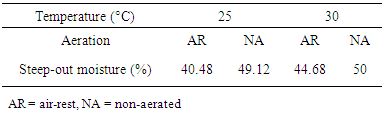
3.2. Steep-out Moisture
- The steep-out moisture content of the grain was affected by both aeration and temperature. There was a general increase in steep-out moisture with increasing temperature. The maximum steep-out moisture was 50% obtained in grains steeped for 24 h under non-aerared (NA), 30°C conditions (Table 1). In their research on a reassessment of sorghum for lager-beer brewing, Agu and Palmer [10] reported that the out-of-steep moisture of sorghum was lower than expected (33–36%) and they discussed that the percentage was adequate for enzymatic modification of the endosperm substrate of sorghum, producing sufficient amylolytic enzymes for brewing lager-type beers. Dewar et al. [8] mentioned that although the absolute steep-out moisture may vary depending on the grain size and cultivar. Generally, the higher the steep-out moisture the better the quality malt produced. The quality of the sorghum malt was found to be directly related to the steep-out moisture of the grain. Aeration during steeping was not shown to be necessary to maximize malt quality.
|
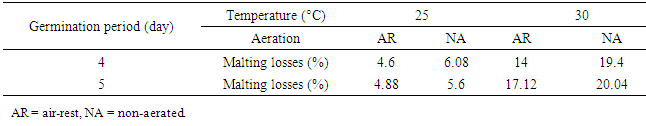
3.3. Malting Loss
- Malting loss was affected by germination period, temperature and aeration. Temperature had greater effect on malting loss than that of germination period and aeration. From the results (Table 1) malting loss for the grains germinated for 4 or 5 days under NA or AR at 30°C was more three times than that of malting loss for the grains germinated for 4 or 5 days under NA or AR at 25°C. However, malting loss varied from 4.6% to 20.04%. The highest malting loss was obtained with grains germinated for 5 days under NA, 30°C conditions (Table 2). Malting losses were generally retarded steeping the grain for increasing periods in non-aerated water but the higher the steeping temperature the higher the losses occurred [8]. The increased malting loss is a characteristic feature of seedling growth and malting [11] [12]. Iwuoha and Aina [13] found that the greatest malting loss was 46.2% in 6 days germination of Nigerian local red sorghum. Owuama and Asheno [14] reported that percentage malting losses from malting grains steeped for 12-24 h and kilned at different temperature regimes vary with sorghum varieties, kilning temperatures and steeping time. Abiodun [15] observed in his investigation of malting properties of twelve sorghum cultivars, the malting loss was increased with the germination period and The malting loss were ranged between 15.5% and 33.9%. Owuama and Adeyema [16] found that for four different Nigerian sorghum varieties, they had different malting losses ranged from 5.4% to 10.6%.
|
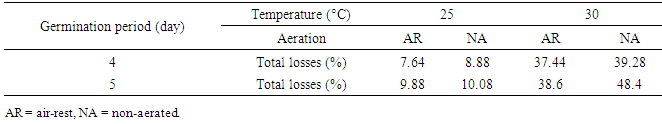
3.4. Total Loss
- The total malting loss varied from 7.64% to 48.4%. The total malting loss was higher with the grains germinated for 5 days under NA, 30°C conditions (Table 3). Total malting loss was mainly affected by temperature. For example, total loss of the grain germinated for 4 days at 25°C under AR condition was 7.64% whilst total loss of the grain germinated for the same time and aeration condition at 30°C was 37.44%.
|
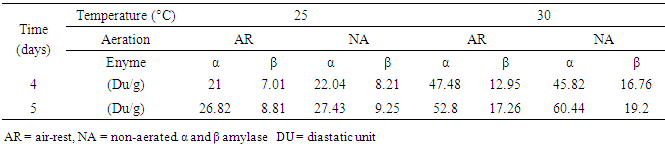
3.5. Diastatic Activity
- The diastatic activity of malted grain ranged from 28 DU/g to 79.84 DU/g (Table 4). Malted grain germinated for 5 days under NA, 30°C conditions showed high DU/g value, whereas malted grain germinated for 4 days under AR, 25°C conditions showed low diastatic activity. Diastatic activity as malting loss was affected by germination period, temperature and aeration. Also temperature had greater effect on diastatic activity than that of germination period and aeration. From the results diastatic activities for the grains germinated for 4 and 5 days under NA and AR at 30°C were more twice than that of diastatic activities for the grains germinated for 4 and 5 days under NA and AR at 25°C. Balogun et al. [17] reported that increasing the temperature during active germination enhanced the hydrolytic activities of endogenous enzymes present in sorghum grain. Dicko et. al. [18] mentioned that red sorghum grains have generally higher amylase activities than white ones. Amylases are hydrolytic enzymes, which depolymerize starch according to a classic acid-base mechanism. α-amylases are endo-enzymes that randomly split α-(1→4)-linkages in starch with retention of anomeric configuration of glucose residues. ß-Amylase is an exoglucosidase acting from the non- reducing end, releasing ß-maltose units from starch, hence the name ß-amylase [19].
|
3.6. α and β Amylases
- α and β amylases were affected by germination period, temperature and aeration. Temperature also had greater effect on α and β amylases than that of germination period and aeration. α and β amylases of the grains germinated for 4 or 5 days under NA or AR at 30°C was increased two folds than that of α and β amylases of the grains germinated for the same periods and aeration conditions at 25°C (Table 5). α- amylase activity of malted grains ranged from 21 DU/g to 60.44 DU/g . β amylase activity ranged from 7.01 DU/g to 19.2 DU/g. Malted grain germinated for 4 days under AR, 30°C conditions showed high proportion of α amylase to β amylase activity which was 3.6 : 1. Whereas, malted grain germinated for 4 days under NR, 25°C conditions showed low proportion of α amylase to β amylase activity which was 2.6 : 1.
|
4. Conclusions
- Sorghum malt quality is affected by steeping temperature. Non-aeration during steeping was also shown to be necessary to maximize malt quality. The quality of sorghum malt was found to be directly related to the steep-out moisture of the grain.For Feterita grain, germination temperature and germination time influenced the response of the grain to the germination process. It appears that increasing the temperature and time during active germination enhanced the hydrolytic activities of endogenous enzymes present in the grain. Malted grain germinated for 5 days at 30°C conditions exhibited relatively better malting characteristics than the grain germinated under other condition as functions of steeping condition and germination time. Further research is needed to use another type of malting scheme to maximize Feterita malt quality and to employ genetic engineering to improve endogenous enzymes of sorghum.
ACKNOWLEDGEMENTS
- The authors express their sincere gratitude to the Ministry of Higher Education and Scientific Research, Sudan for financing this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML