-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2016; 6(4): 73-80
doi:10.5923/j.food.20160604.01

Nutritional, Sensory and Storage Quality of Sekete from Zea mays.
Obatolu V. A., Adeniyi P. O., Ashaye O. A.
Institute of Agricultural Research and Training, Apata, Ibadan
Correspondence to: Adeniyi P. O., Institute of Agricultural Research and Training, Apata, Ibadan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Many of our cultural values have been going into extinct including the nutritionally related habits. Rejuvenating these could enhance the application of dietary diversification towards achieving nutrition security. Sekete is one of the traditional beverages in Nigeria that is no longer commonly produced nor consumed, hence, a deep understanding of its nutritional, sensory and storage quality may promote its consumption and even commercialization. This study was therefore designed to determine the nutritional, sensory and storage quality of Sekete from Zea mays. Sekete was prepared from yellow maize using traditional method and was divided into pasteurized (at 65°C for 30 minutes), unpasteurized plain, sweetened and fortified (with soymilk in ratio 3:1 v/v). Samples were analyzed (1st day and at 4 weeks of cold storage (4°C) for proximate composition, antioxidant capacity, thiamine, riboflavin, niacin, ethanol, pH as well as microbial analyses (total viable, coliform, total fungal and total anaerobic count) with the isolation of the microbes using standard methods. Mean data were analyzed using Duncan’s Multiple Range Test at p≤0.05. Processing of the maize into Sekete did not significantly improve the nutritional composition, pasteurization significantly lowered the antioxidant capacity in all the samples but storage significantly increased this parameter. Fortification and sweetening significantly increased the nutritional and sensory properties of the drink. Bacillus and Lactobacillus were isolated and the samples were free of coliforms even after storage. The pH ranged from 2.9 to 3.0 (unfortified) and 3.4 to 3.5 in fortified compared with 7.0 in the control. Fortification and sweetening improved the nutritional and sensory properties of Sekete and storage at refrigerating temperature for 4 weeks preserved the drink. Fortified and sweetened Sekete production for household consumption and commercial purpose is hereby recommended.
Keywords: Sekete, Nutritional quality, Sensory evaluation, Storage quality
Cite this paper: Obatolu V. A., Adeniyi P. O., Ashaye O. A., Nutritional, Sensory and Storage Quality of Sekete from Zea mays., International Journal of Food Science and Nutrition Engineering, Vol. 6 No. 4, 2016, pp. 73-80. doi: 10.5923/j.food.20160604.01.
Article Outline
1. Introduction
- Dietary diversification is a strategy for improving food and nutrition security by making variety of foods available at individual, household and national levels. Possible dietary diversification techniques or activities include: promotion of mixed cropping and integrated farming systems; introduction of new crops (for example soybeans); promotion of underexploited traditional foods and home gardens; small livestock raising; promotion of fishery and forestry products for household consumption; promotion of improved preservation and storage of fruits and vegetables to reduce wastage, post-harvest losses and effect of seasonality; strengthening of small scale agro-processing and food industries; income generation and nutrition education to encourage the consumption of healthy and nutritious diet year round (www.fao.org). Application of any of these is capable of enhancing food security at all levels. Traditional food crops are the crops accepted by a community through habit and tradition as appropriate and desirable sources of food. People are accustomed to them; they know how to cultivate and prepare them and enjoy eating dishes made from them. However, these may be grouped into two major categories: those that are consumed as traditional dietary staples such as cassava, yam, plantain, sweet potato, millet, sorghum, maize, beans; and those that serve as ingredients in accompanying the staples in soups, sauces, stews, and drinks such as legumes, oilseeds, melon, fruits and vegetables. In Nigeria it is traditional to use cereals to prepare drinks. Sorghum is used in brewing beer for different cultural festivities such as meetings, weddings, birthdays, naming ceremonies, cultural festivals and so on. In some parts of the country over ripe plantain is used while some parts use maize, bark of fruit trees, tea leaves to prepare traditional drinks. Dietary diversification increases the margin of food choices and presents a wide variety of foods from which individual choices can be made. The rejuvenation of our traditional foods and drinks which have been going into extinct could be a laudable and feasible intent towards achieving food security especially among the rural poor. Burukutu, pito, kununzaki and zobo are indigenous to the northern part of the Nigeria, Sekete to the south western part while adoyo is indigenous to Ilorin also in south western part of the country. Burukutu, pito and kununzaki are prepared from sorghum, zobo drink from roselle, sekete from maize, adoyo from ripe pineapple and supernatant from ogi (fermented slurry from maize or sorghum). Pito can also be prepared from tea leaf (Camelia species), cashew tree bark (Anacardium occidentale) or bark of mango tree (Mangigera indica). Unfermented pito combined with adoyo may be served as a drink but more importantly for medicinal purposes. All these are traditional or indigenous beverages which may be taken for stimulating, nourishing, refreshing, or medicinal effect especially among the rural and urban poor people. There is therefore need to promote the consumption of these beverages. In the advent of civilization, the preparation and consumption of some of these traditional drinks has gradually gone into extinct as in the case of Sekete. Sekete is a traditional drink commonly prepared from sprouted maize. In Nigeria other traditional beverages consumed are pito, kununzaki, burukutu, zobo drink and so on. While burukutu is basically an alcoholic beverage, pito and zobo are refreshing and kununzaki is a nourishing drink. Sekete has also been identified as a local fermented drink and very few scientific studies have been reported on it. Decrease in phosphorous, magnesium, potassium and sodium was observed in the beverage after fermentation, riboflavin and niacin contents increased and thiamine decreased slightly at the end of fermentation, an increase was observed in nitrogen and protein content but the ash and crude fibre decreased (Sanni, 1989). Sekete was reported to be a rich source of important amino acids such as alanine, leucine, tyrosine and lysine but lacked proline and arginine (Fapohunda, 1988). The isolated microfungi from Sekete include Aspergillus flavus, Cladosporium cladosporioides and Alternaria tenuissima (Fapohunda, 1988). Pasteurization of Sekete at different temperatures was observed to reduce the microbial load and increase the keeping quality of the beverage. Samples pasteurized at 65°C for 30 minutes had a bacterial load increasing from 4.0× 101 (first week) to 2.0× 102 cfu/ml (fourth week), samples pasteurized at 70°C for 30 minutes had a bacterial load ranging from 1.0 × 10 cfu/ml (first week) to 2.0 ×10 cfu/ml (fourth week) while there was no bacterial growth in the samples pasteurized at 75°C for 30 minutes (Onaolapo and Busari, 2014). The samples were free of yeast growth throughout the storage periods and the samples were not significantly different in sensory properties except in taste (Onaolapo and Busari, 2014). Microorganisms isolated from Sekete, pito and burukutu were Saccaromyces cerevisiae, Acetobacter aceti, A. hanseni, A. pasteurianus, Alcaligenes, Flavobacterium, Lactobacillus plantarum and L. brevis (Sanni et al., 1999). There was also observed a decrease in ethanol from 3% in fresh products to 1% at the end of 72 hour retailing period and this was proportional to the acetic acid content of the drinks, however, the acid constituents of the drinks (lactic, malic, succinic and formic acids) witnessed a slight decrease. Even though no enterobacteriacieae was isolated some of the identified isolates are potentially pathogenic and may predispose consumers to food infection (Sanni et al., 1999).Current in depth understanding of the nutritional, sensory and storage quality of sekete is a needed step in promoting the consumption and commercialization of the traditional beverage, hence, this study aimed at determining the nutritional, sensory and storage quality of sekete from Zea mays.
2. Materials and Method
- Collection of maizeYellow maize grains (DMR-LSR-Y) was obtained from the seed store of the Institute of Agricultural Research and Training, Apata, Ibadan. These were cleaned to remove broken grains and foreign materials.Preparation of SeketeThe traditional method of preparing sekete as described by Adegoke et al, 1995 was used with slight modification. Maize was soaked in clean water for 24 hours after which the water was drained and the grains spread on wet muslin cloth. This was wet with water every day to sprout for 3 days. The sprouted grains were washed and wet-milled after which the slurry was diluted with clean water in the ratio 2:5 (v/v). This was covered in a plastic jar and allowed to ferment for 2 days after which it was sieved. This was then boiled for 30 minutes to obtain the plain alcoholic sekete sample. Half of this was boiled for extra 1 hour and designated as the non-alcoholic sekete sample.Pasteurized and unpasteurized samplesSamples to be pasteurized were bottled in heat resistant plastic bottles while the unpasteurized samples were bottled in plain transparent PET bottles. Pasteurization was achieved by heating the sample at 65°C for 30 minutes after which cooling was done rapidly. To sweetened samples table sugar (sucrose) was added at 50g/L while to the fortified samples soymilk was added in ration 1:3 (v/v). Control sample was obtained by wet-milling the maize, diluting the slurry in ratio 2:5 and boiling for 30 minutes. Samples were designated as follows:RCS--raw control samplePCS–pasteurized control sampleUCS–unpasteurized control samplePSNA–Pasteurized sweetened Non-alcoholic (NA) sampleUSNA–Unpasteurized sweetened NA samplePFNA–Pasteurized fortified NAUFNA–Unpasteurized fortified NAPPNA–Pasteurized plain NAUPNA–Unpasteurized plain NAPFA–Pasteurized fortified AlcoholicUFA–Unpasteurized fortified AlcoholicPPA–Pasteurized plain alcoholicUPA– Unpasteurized plain alcoholic.Proximate compositionSamples were analyzed for proximate composition, thiamine, riboflavin, niacin, ethanol, antioxidant capacity and pH at the day of production and at the 4th week of storage at 4°C. Microbial analysis (total anaerobic count, total aerobic count, coliform count and fungal count) was done weekly. The method of A.O.A.C, 2005 was used to determine the protein, fat, crude fiber, ash content and carbohydrate was determined by difference.Plate Count or microbial count using The Pour Plate Method (Harrigan Macanee, 1976)One (l) ml of the sample was aseptically dispensed into a sterile petri dish using a sterile pipette. A measured quantity (15 - 20ml) of sterile nutrient agar was added and the two mixed thoroughly by swirling gently. The dish was then incubated at 37°C for 18-24hours. The number of colonies growing in the agar plate was then counted.Determination of antioxidant capacity (Free Radical Scavenging Activity)The method of A.O.A.C. 2005 was used. Method is based on measurement of loss of color of DPPH solution by the change of absorbance at 517nm caused by the reaction of DPPH with the test sample. To 0.2ml of the sample 2.8ml of freshly prepared 20mg/dm3 DPPH in Methanol was added. This was incubated for 20min at room temperature. Inhibition in % RSC (Radical Scavenging Capacity) was calculated as follows:RSC % = (1 - Asample/Ablank) x 100%Ablank is Abs for Control while Asample is Abs for test sample.Determination of B vitaminsRiboflavin, Thiamine and Niacin The Spectrophotometric method of the Association of Official Analytical Chemists (2005) was used.RiboflavinTo 0.5g of the sample 30ml Dichloroethane and 30ml of 30% HCl (ratio1:1) were added after which 50ml Ammonium Hydroxide solution was added. This was filtered and the absorbance was read using a spectrophotometer (Thermo Scientific Spectronic 20 model, United States) at 415nm.Preparation of standard curveFrom the stock solution of standard Riboflavin, 0.1- 0.5 ppm solutions were prepared and the absorbance was read using the spectrophotometer. These were then used to plot the standard curve which was used to extrapolate the results of the readings for the samples.ThiamineA small quantity of the sample (1g) was weighed and 50ml of 50% methanol and 50ml of 17% sodium carbonate were added (ratio 1:1) in order to extract the vitamin. This was then filtered and Follins-Denis reagent was added to the filterate. This was allowed to cool until it gives a blue colour. The absorbance was then read at 415nm with a spectrophotometer. A standard curve was constructed using the absorbance values of calibrators with known thiamine content which was used to extrapolate the thiamine content of the samples.NiacinNiacin was extracted from the food by autoclaving the sample with aqueous calcium hydroxide. A small portion (1g) of food sample was mixed with 0.75g of calcium hydroxide in centrifuge tubes and 20ml of deionized water was added. The mixture was thoroughly mixed and heated in an autoclave for 30 minutes at 121°C with the centrifuge tube cap loosened. This was followed by dilution to 50ml with distilled water then mixing and was allowed to cool. The mixture was then centrifuged at 0°C, 2500rpm for 15 minutes. The pH of the supernatant (15ml) was adjusted to 7 with aqueous oxalic acid and made to 25ml with distilled water in a vial. The resultant suspension was centrifuged at 2500 rpm for 10 minutes to precipitate the calcium oxalate. The absorbance was read at 650nm.Preparation of Standard CurveThe blank which contained all the reagents used in the extraction was prepared. The standard solutions of reference niacin at 1 to 10 ppm were also prepared and 1ml of cyanogen bromide per ml was added to each. The absorbance of these standard solutions was read at 650nm and these were used to plot a standard curve. The niacin contents of the samples were then extrapolated from the standard curve. Determination of EthanolThe method of A.O.A.C, 2005 was used. Into a volumetric flask 25ml of sample was measured at 20°C. This was washed into a separator with 100ml distilled water. The mixture was saturated with NaCl and shaking with 100ml light petroleum ether for 3 minutes was done. This was allowed to stand for at least 15 minutes after which the lower layer was run into a distillation flask. The light petroleum extract in the separator was shaken with 25ml saturated NaCl solution after allowing the mixture to separate. The solution was made just alkaline with 1N NaOH after adding solid phenolphthalein. Pumine was added and then 100ml distilled water after which distillation of 90ml of the mixture was done into a 100ml volumetric flask. This was then made up to the mark with water at 25°C and the specific gravity and refractive index were determined. Sensory evaluationSamples were given to a 20-member trained taste panel of judges. A 9-point hedonic scale was used with 1 designated as ‘dislike extremely’ and 9 designated as ‘like extremely’. The panelists were asked to evaluate the samples for appearance, flavor, color, mouthfeel, taste, aftertaste and overall acceptability (Ihekoronye and Ngoddy, 1985). The scores were subjected to Analysis of Variance using Duncan’s Multiple Range Test to signify significant difference (p≤0.05). Statistical AnalysesAnalyses were carried out in triplicates and mean data were analyzed using Duncan Multiple Range Test with SPSS version 17.
3. Result and Discussion
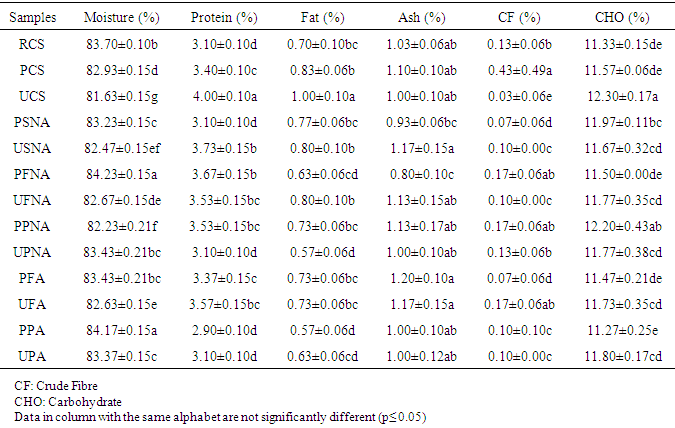
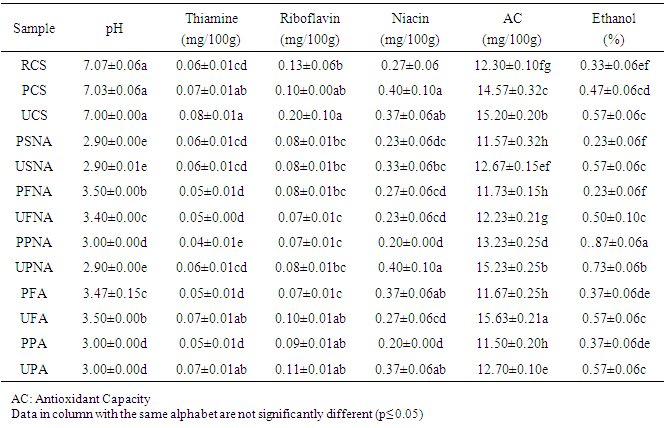
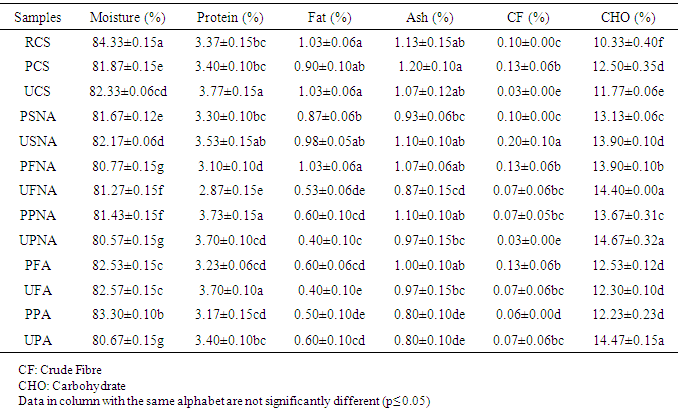
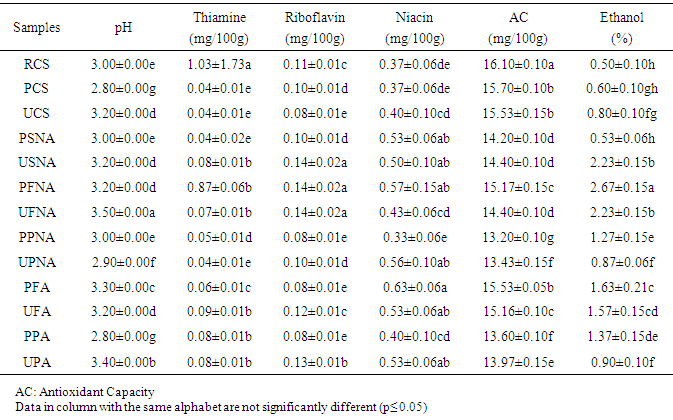
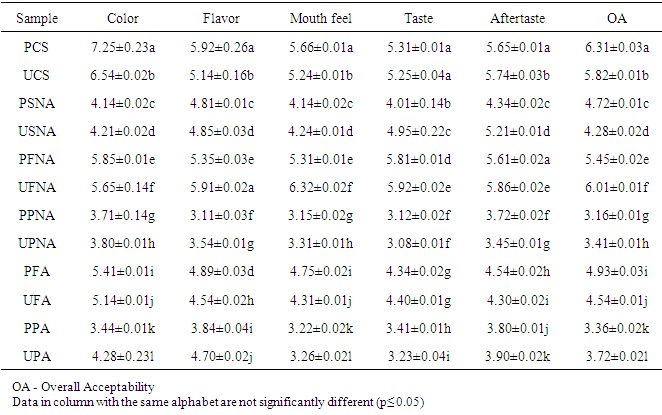
- The proximate composition of the different sekete samples are expressed in Table 1.
|
|
|
|
|
4. Conclusions and Recommendations
- Sekete is a high acid local beverage from maize which is rich in Lactobacillus species and Saccharomyces species and the fermentation was more of a lactic acid fermentation rather than saccharification. Fortification improved the nutritional composition of the beverage and it increased the pH of the samples while 4 weeks of cold storage increased the antioxidants and alcohol content. The cold storage did not adversely alter the nutritional composition of the beverage. The sekete samples were still free of coliforms at the end of 4 weeks of cold storage (4°C). While pasteurization reduced the antioxidant capacity in all the samples it did not alter other components. The relaxative, medicinal and stimulating effect commonly experienced after sekete consumption may be a result of the microbial profile, acid composition and the activities of the microbes. Also, fortification and sweetening increased the overall acceptability of the beverage, hence, fortified and sweetened sekete is recommended for commercial and household production and consumption. However, quantification and identification of the acid component of sekete is recommended as well as the storage quality beyond 4 weeks cold storage.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML