-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2016; 6(1): 1-8
doi:10.5923/j.food.20160601.01

Nutritional Characterization of the Long-whiskered Catfish Sperata aor: A Commercially Important Freshwater Fish of Bangladesh
Sayad Md. Didarul Alam, Md. Hasanul Karim, Aninda Chakrabortty, Ruhul Amin, Sohel Hasan
Department of Biochemistry and Molecular Biology, University of Rajshahi, Rajshahi, Bangladesh
Correspondence to: Sayad Md. Didarul Alam, Department of Biochemistry and Molecular Biology, University of Rajshahi, Rajshahi, Bangladesh.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The freshwater catfish (Sperataaor) contributes significantly to the fisheries production in the tropical rivers and also enjoys high consumer preference. The present study was to generate evidence on the nutrient profile of long-whiskered catfish S.aor as such information is not available. Fatty acid profiling showed that S.aor is enriched in omega-3 polyunsaturated fatty acids. The fatty acids in Sperataaor were found to be oleic acid (22.37%), palmitic acid (21.96%), linoleic acid (7.20%) myristic acid (6.95%) and stearic acid (6.46%). Amino acid analysis showed that the fish flesh is rich in the essential amino acids like histidine, threonine and leucine and the ratio of essential to non-essential amino acid is 0.86 indicating its superior protein quality. The mineral profiles showed that the fish is rich in sodium, potassium, calcium and magnesium. The present study also showed that Sperataaor is a good source of lean meat, trace elements (especially zinc and iron) and fat soluble vitamins A, D, E and K.
Keywords: Freshwater catfish, Sperataaor,Amino acids, Fatty acids, Micronutrients, Nutrient profile
Cite this paper: Sayad Md. Didarul Alam, Md. Hasanul Karim, Aninda Chakrabortty, Ruhul Amin, Sohel Hasan, Nutritional Characterization of the Long-whiskered Catfish Sperata aor: A Commercially Important Freshwater Fish of Bangladesh, International Journal of Food Science and Nutrition Engineering, Vol. 6 No. 1, 2016, pp. 1-8. doi: 10.5923/j.food.20160601.01.
Article Outline
1. Introduction
- Bangladesh is one of the largest deltas in the world, second to the Amazon located at the head of the Bay of Bengal, a northern extended arm of the Indian Ocean. The country is a land of rivers and act as a drainage outlet for a vast river basin complex made up of the Ganges-Brahmaputra-Meghna river system, and rich in various fisheries resources [1].The pride of Bangladesh is its rivers with one of the largest network in the world with a total number of about 230 rivers which have a total length of about 24,140 km [2]. The inland fishery resources of Bangladesh are considered as highly potential resources of the world. Inland waters which include River, Lake, Pond, Beel, Haor, Baor, Floodplain, Estuary, Canal etc. cover about 46,99,345 ha, which act as a main source of natural habitat for commercially important freshwater fishes of the country. All these water bodies offer tremendous scope and potential source of fisheries. According to DoF 2013, about 9,57,095 MT and 17,26,067 MT of total catch were obtained from capture fishery and culture fishery, respectively [3].Fish is one of the cheapest and available sources of animal protein and is an important dietary constituent. Fish alone supplies about 60 percent of animal protein and about 10.5 million people are directly or indirectly earn their livelihood out of activity related to fisheries [4]. A number of regional studies have confirmed the significance of fish items in a Bangladeshi diet [5, 6]. Fish plays an important role in prevention of the chronic protein-calorie malnutrition diseases such as kwashiorkor and marasmus. Fish is often promoted as a healthy part of the human diet due to its high content of long chain n-3 polyunsaturated fatty acids (LC-PUFA). The most significant benefits of fish oil especially from marine species, is the richness of ω-3 polyunsaturated fatty acids (PUFAs) particularly eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6) [7] which combat some diseases such as asthma, multiple sclerosis and systemic lupus erythematosus [8, 9]. These two fatty acids must be obtained from the diet as human body cannot synthesize thus called essential fatty acid. Oil from fish species is linked with numbers of health benefits and prevents cardio-vascular diseases (CVDs) [10]. In addition, fish oil plays vital role in remediation of many diseases and is a protective mean of various types of abnormalities such as diabetes mellitus, cancers, inflammation, hypertension, obesity, rheumatoid arthritis, osteoporosis and schizophrenia. Also, the fish goes to high protein, minerals and low lipid group. They contain lower caloric content per unit of protein compared to lipid and they are an ideal source of animal protein for use in controlling diets [11]. Fish is also an important source of macro and micronutrients [12, 13]. Fish oil is an exceptional source of vitamins A, D, E and K. Vitamin A and D are essential for healthy skin and for the improvement of bones. Vitamin D plays an important role in the body’s use of calcium and mineral is vital for sound teeth and bones. Furthermore, fat fish in particular are the major source of vitamin D [14]. It is very crucial to generate evidence on nutritional composition of fish as it can broaden our insight about information on precise nutrient abundance of particular species which will be helpful for the nutritionists and dieticians to design a proper ‘dietary guidelines’. The long-whiskered catfish S. aor (Actinopterygii: Siluriformes: Bagridae) is locally known as “Ayre or Aor” in Bangladesh [15]. It is one of the commercially important freshwater catfish, and was once abundant in canals, rivers, floodplains, inundated fields, swamps, ditches, ponds and reservoirs in Bangladesh [15]. It has been considered as one of the most admired edible fishes due to low number of intramuscular bones and high nutritional value with good protein content [16].In the present study, oil and flesh were investigated from Sperata aor to define the nutrient contents. Several studies have been carried out on the effects of consumption of nutrient dense fresh water fishes [13, 17] and it is very important to make relationship between human nutrition and fisheries [12]. Proximate composition analyses of Sperata seenghalaa (belongs to the same genus with Sperata aor) have been already reported [18, 19]. Sperata seenghala is almost alike with Sperata aor, it has been considered as one of the most admired edible fishes due to its good taste and low number of intramuscular bones. It has high nutritional value with good protein content; each gram of its flesh contains 200 unit of vitamin A. It is a popular species of catfish to capture because it fetches a higher price than carp [20]. But due to physiological, geographical and ecological factors, these values vary widely. Therefore, in the present study, the assessment of different nutritional parameters such as amino acid, fatty acid, mineral and vitamin profiles long-whiskered catfish Sperata aor was taken into account.
2. Materials and Methods
2.1. Sample Collection and Preparation
- Due to their availability in all seasons throughout the country, Ayre fishes were collected from the local market in Rajshahi, Bangladesh. The samples were cleaned and washed with demineralized water. Only the flesh samples after removal of internal organs, head, skin and tails, were taken out from the body. Samples were packed in polyethylene bags to store in a refrigerator (at 4ºC) for experimental purposes for 24 hrs.
2.2. Extraction of Oil from Fish
- The stored flesh samples were sun-dried for about 72 hrs and grinded by mechanical grinder. Oil was extracted by Soxhlet extraction apparatus using n-hexane and stored at 4ºC for further analysis [21].
2.3. Fatty Acid Composition Analysis and GC Conditions
- To determine the fatty acid composition of oil, fatty acid methyl esters (FAMEs) were prepared using standard AOCS [22] method. FAMEs were quantified by Gas Chromatograph (GC). A Hewlett Packard GC (Model: 6890 Series II Gas Chromatography System) was used. The column was a fused silica capillary column (100 m length x 0.25 mm diameter, 0.2 μm of film, Supelco, Bellefonte, Pennsylvania, USA). The oven temperature was set according to Uddin et al. [23]. The primary temperature, 130°C was kept constant for 3 min and then allowed to increase into 240°C at a rate of 4°C/min followed by a hold at 240°C for 10 min. Nitrogen gas was used as the carrier gas of the fatty acid methyl esters with flow rate of 1 mL/min. The injector and detector temperature were maintained at 250°C. FAMEs were identified by comparison with the retention times of standards from Supelco (Pennsylvania, USA) and quantified by comparing with respective peak areas.
2.4. Nutritional Quality Index (NQI) of Lipids
- The nutritional quality of the lipids was assessed by considering three indexes: atherogenicity (AI), thrombogenicity (TI) and the ratio between the hypocholesteronic and hypercholesteronic. The following calculations were used to determine these indexes: a) Atherogenicity Index [24] (AI) = [(C12:0 + 4xC14:0+C16:0)]/ΣMUFA+Σn-6+Σn-3 b) Thrombogenicity Index [24] (TI) = (C14:0 + C16:0 +C18:0)/[(0.5×ΣMUFA)+(0.5×∑PUFA ω-6)+(3x∑PUFA ω-3)+( ∑PUFA ω-3/∑PUFA ω-6)] c) Ratio between hypocholesterolemic and hypercholesterolemic fatty acids [25]. (HH) = (C18:1n-9+C18:2n-6+C20:4n-6+C18:3n-3- + C20:5n-3+C22:5n-3+C22:6n-6)/(C14:0+C16:0)
2.5. Amino Acid Composition
- Acid hydrolyses: In order to determine amino acid profiles, samples were hydrolyzed at 110°C under nitrogen atmosphere for 24 h with 6.0 mol/L hydrochloric acid. Amino acids were analyzed by HPLC using amino acid analyser LC 10AS (Shimadzu, Japan) [26]. The column used was C-18 polystyrene column. FL 6A fluorescence detector was used with CR6A Chrom Pac recorder software. The analyzer was calibrated using amino acid standard solutions (Sigma-Aldrich, A2161-5ML) and the same solutions were used to calculate the amount of amino acids in the samples.Alkaline hydrolysis: For the tryptophan analysis, samples were digested with 5% NaOH for 24 h and were then neutralized to pH 7.0 with 6N HCl. Tryptophan content was measured spectrophotometrically at 530 nm as per Sastry and Tammuru [27].
2.6. Estimation of Quality of the Amino Acids
- The total amino acid (TAA), total essential amino acids (TEAA), total acidic amino acid (TAAA), total sulfur amino acids (TSAA), and total aromatic amino acids (TArAA) were calculated from quantity of amino acids. The predicted protein efficiency ratio (PER) was determined using the equation developed by Adeyeye (2009) [28]:PER=−0.468+0.454(Leucine)−0.105 (Tyrosine)The amino acid score (AAscore) for the essential amino acids was calculated using the following formula [29]:AAscore=AAASP/AAARpWhere AAASP is the amount of amino acid per sample protein (mg/g); AAARP is the amount of amino acid per protein in reference protein (whole hen’s egg) (mg/g).
2.7. Mineral Composition Analysis
- The trace elements (Ca, Mg, Zn, Fe, Hg, Pb, and Cr) were analysed in “AAS-680” Atomic Absorption / Flame Emission Spectrophotometer (Shimadzu, Japan) with a single hollow cathode lamp for each element having an air-acetylene and nitrous-oxide-acetylene [30]. Analytical grade reagents of respective salts were used to make stock standard solutions of 100 ppm with deionized water and preserved in clean polythene bottles. Stock standard solution was used to prepare standard solutions of these metal ions by suitable dilution using deionized water. Also, the fish sample was diluted to a known volume and analysed by a Flame AAS. The sample was analysed against standard solution of each element. A blank reagent was also continued, and the absorption due to reagent was subtracted. Quantification was done by comparing with multi elemental standard.
2.8. Assessment of Vitamins
- About 150 mg fish oil was refluxed with 25 mL methanol and 150 % potassium hydroxide (KOH) in water bath for 30 min. Fat soluble vitamins were extracted using 50 mL petroleum ether. The petroleum ether layer was collected, concentrated and dissolved in 5 mL of acetonitrile. Fat soluble vitamins were analysed by HPLC (Shimadzu LC 10AS, Japan). The C18 RP column and UV detector were used. The injection volume was 20 µL. Acetonitrile (solvent A) and methanol (solvent B) were used as mobile phase and the flow rate was 1 mL/min. A simple linear gradient system was used, starting from (solvent A/solvent B) 50/50 to 70/30 in 20 min. The fat soluble vitamins were identified and quantified by comparing with the retention times and peak area of respective vitamins standards (Sigma-Aldrich) [31].
2.9. Statistical Analysis
- The values were subjected to the statistics variance analysis (ANOVA). The Tukey’s test was applied to the unequal variances among the average sample values, keeping the significance level in 5% in all analyses through the software.
3. Results and Discussion
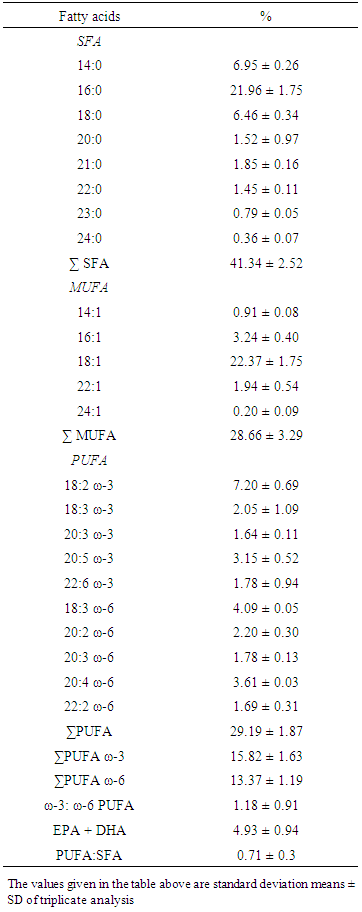
3.1. Fatty Acids Profile
- Freshwater and marine fish are often considered to be a healthy component of the human diet, due to relatively high ratios of polyunsaturated to saturated fatty acids (PUFA:SAFA) compared to other animal food sources [32]. In particular, fish contain high concentrations of ω-3 long-chain PUFA (LC-PUFA), such as eicosapentaenoic acid (EPA, 20:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3). These fatty acids have been identified as essential elements of the human diet [33] because they cannot be synthesized in amounts adequate for optimal health [34]. These essential ω-3 fatty acids have been, and continue to be, investigated extensively in health studies, where the benefits of dietary consumption of ω-3 LC-PUFA have been found in relation to cardiovascular disease, diabetes, inflammatory diseases, and neurological/neuropsychiatric disorders [35]. Nutritional guidelines from various health agencies worldwide now provide recommendations for the dietary intake of EPA + DHA and/or ω-3 fatty acids (e.g., [36, 37, 38, and 39]. In addition, diets with ω-3: ω-6 ratios close to 1 [38, 40] and PUFA:SFA ratios >0.4[41] are also recommended for optimal health. Health Canada (2011) recommends the consumption of at least 150 g of cooked fish each week as part of a healthy diet [32].Fatty acid compositions of muscle tissues of S. aor are presented (Table 1). The percentage of the total polyunsaturated fatty acid in oil was 34.99%. The important polyunsaturated fatty acids EPA and DHA were found to be 3.15 ± 0.52% and 1.78 ± 0.94% in oil, respectively. These values were lower than the value of 7.7% EPA and 14.5% DHA for salmon fish oil [42]. Among the monounsaturated fatty acids, C18:1 (22.37 ± 1.75%) was present in higher amounts in oil. The most significant saturated fatty acid was C16:0 (21.96 ± 1.75%). The total SFA, MUFA, PUFA, ω-3: ω-6, PUFA:SFA of S. aor were also calculated and presented on table -1.
|
3.2. Nutritional Quality Index (NQI)
- Indexes of the nutritional quality of the lipid was determined by Atherogenicity Index (AI), thrombogenicity Index (TI) and ratio between hypocholesterolemic and hypercholesterolemic fatty acids (HH) and found to be 0.86 ± 0.03, 0.51 ± 0.07 and 1.14 ± 0.18, respectively.
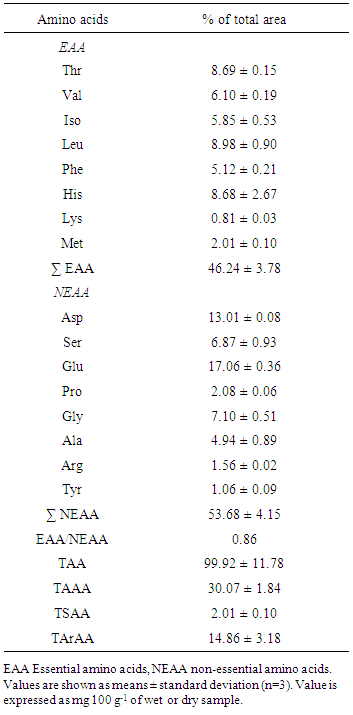
3.3. Amino Acids
- Amino acids are the building blocks of body proteins and responsible for growth, development, repair as well as maintenance of cell [43]. Dietary protein plays important role providing amino acids for the biosynthesis of the body proteins. It is very important to supply all essential amino acids to the tissues in an appropriate amount for optimal protein synthesis. Fish proteins comprise all the essential amino acids vital for human nutrition which expand the overall protein quality of a diet [44].The amino acid profile of S. aor is presented in Table 2. All the data given in the table have been expressed in percentage of total area of amino acids. The principal amino acids found in S. aor flesh are glutamic acid (17.06 ± 0.36%) followed by aspartic acid (13.01 ± 0.08%), leucine (8.98 ± 0.90%), threonine (8.69 ± 0.15%) and histidine (8.68 ± 2.67%) which have a close link with previous results found in S. seenghala [45]. Amino acids like lysine (0.81 ± 0.03%), tyrosine (1.06 ± 0.09%), arginine (1.56 ± 0.02%), methionine (2.01 ± 0.10 %) and proline (2.08 ± 0.06 %) were low in amount with respect to other amino acids. S. aor contains higher amount of essential amino acids (EAA) histidine, threonine, isoleucine, valine and leucine than C. striatus [46]. Among the EAA, leucine, threonine and histidine are predominant and the non-essential amino acids (NEAA) like glutamic acid, aspartic acid and serine are found in higher amount.
|
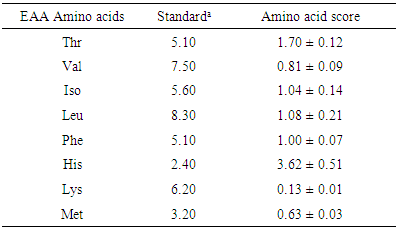
3.4. Estimation of Quality of the Amino Acids
- The protein efficiency ratio (P-PER) was found to be 3.498. Table-3 presents the essential amino acid whole hen’s egg scoring pattern of the fish [49]. It can be deduced from here that the limiting amino acid is lysine, while the most abundant is histidine. Others are also relatively high, which implies that the fish is a good source of protein and amino acids. Lysine has been reported to be the limiting amino acid.
|
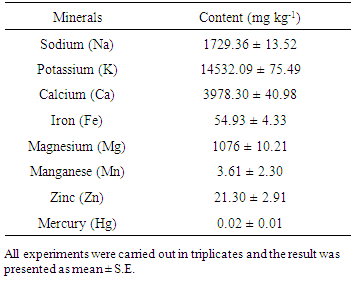
3.5. Minerals
- The mineral contents of S. aor are presented in Table 4. The macro-minerals potassium, calcium and sodium contents were found to be 14532.09 ± 75.49, 3978.30 ± 40.98 and 1729.36±13.52 (mg kg-1 of wet flesh), respectively. These values are closely related to the S. seenghala [45].The potassium content of S. aor is about two times higher compared to Puntius stigma (male 7190.0 mg kg-1, female 6550.0 mg kg-1), a nutrient dense small indigenous fish [50]. Also in case of calcium content, S. aor has higher value compared to the major carps Labeo rohita (862.8 mg kg-1) and Labeo calbasu (3185.0 mg kg-1), respectively [51]. Calcium is essential for normal functioning of muscle, nervous system and strong bones (formation and mineralization). Calcium deficiency leads to the development of rickets in young children and is responsible for osteomalacia (softening of bones) in adults and aged people. So S. aor can be a great supplementary source of calcium.The other macro minerals such as manganese, iron, zinc and magnesium were found to be 1076 ± 10.21, 54.93 ± 4.33, 21.30 ± 2.91 and 3.61 ± 2.30 (mg kg-1 of wet flesh), respectively. Zinc is required for growth, development as well as the proper functioning of immune system and healthy skin. It also has a role in cell division, cell growth, wound healing and metabolism. Deficiency of Zn is associated with skin problems, poor growth and loss of hair with other problems. Compared to the major carps Cirrhinus cirrhosus (15.0 ± 0.1 mg kg-1) [52] and Labeo rohita (0.84 ± 0.22 mg kg-1) [53], S. aor contains higher level of Zn.Moreover, iron is crucial for synthesis of hemoglobin in red blood cells which transports oxygen throughout the body. Iron deficiency is related with anemia, impaired brain function as well as poor learning ability and poor behavior in infants. Its deficiency is also associated with increased risk of infection [54]. Iron content of S. aor is very high compared to the murrel Channa punctatus (20.0 mg kg-1) known for its richness in micronutrients [50]. The high iron content makes this species nutritionally significant. Mercury is a heavy metal which causes mercury toxicity [55]. Mercury content in S. aor to be 0.02 ± 0.01 mg kg-1 which is well within the safety limits, as the prescribed permissible level according to WHO of mercury in fish flesh (0.50 mg kg-1) [56].
|
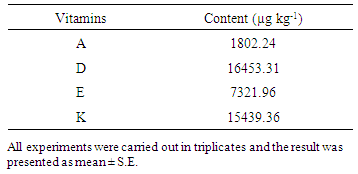
3.6. Vitamins
- Fish is a good source of fat soluble vitamins A, D, E and K. Vitamin A is required for normal vision as well as for bone development and growth. Its retinoic acid regulates gene expression in the development of epithelial tissue [52]. The fat soluble vitamins found in S. aor are presented in Table 5.
|
4. Conclusions
- Nowadays, malnutrition is an alarming concern in developing and under-developed countries. Being one of the inexpensive sources of animal proteins, fish can play a significant role to prevent the diseases associated with protein-calorie malnutrition. Due to tastiness and fewer numbers of intramuscular bones, S. aor is an extremely favored fish species as food. From results, S. aor has highest protein, minerals and vitamins contents, it can be an important source of nutrition. S. aor could be used as a tremendous source of good quality fatty acids, especially PUFA.Finally, the results provide valuable information about to make a proper balanced diet by nutritionist and dietitians. The evidence on nutrient outline of S. aor from this study indicates if this species is brought into aquaculture, it can play a vital role in the prevention of protein-calorie malnutrition in the underprivileged population, including fisherman community.
ACKNOWLEDGEMENTS
- The authors are very thankful to National Institute of Science and Technology, Ministry of Science and Technology, Government of the People’s Republic of Bangladesh for financial assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML