-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2015; 5(5): 203-208
doi:10.5923/j.food.20150505.04
Effect of Redox Agents and Thermo Molding on the Network Formation and Stability of Wheat Gluten Bio-Plastics
Abrehet Fisseha
School of Nutrition, Food Science and Technology, Hawassa University, Hawassa, Ethiopia
Correspondence to: Abrehet Fisseha, School of Nutrition, Food Science and Technology, Hawassa University, Hawassa, Ethiopia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The study was aimed to evaluate the effect of redox agents and thermal treatment on the net work formation and stability of wheat gluten bio-plastic. Gluten was fractionated to gliadin- and glutenin- rich fractions based on the Osborn techniques with ethanol alcohol. Gluten, gliadin-, glutenin-rich and mixing ratio of gliadin-glutenin-rich fractions at 0.25:0.75, 0.5:0.5, and 0.75:0.25 respectively were molded at 130 and 150C during 5 and 25 min. The protein extractability, free SH content, amino acid composition and water absorption capacity of the molded samples was determined. The extractability, free SH content and water absorption was significantly affected by the molding condition, composition and presence of redox agents. The increased glutenin concentration results lower extractability and water absorption capacity and increased the free SH content before and after thermo-molding. With the thermal treatment; the extractability and water absorption capacity was highly reduced while the free SH content was increased as amino acids (cystine and lysine) were responsible for the network formation in the bio-plastics. The higher decrement of the protein extractability with increased glutenin content and molding condition explains the susceptibility of glutenin. The lower water absorption capacity of glutenin before and after molding was an indication of consistent cross-linking formation.
Keywords: Protein Extractability, Redox agents, Free SH, Thermo-molding, Amino Acids, Water absorption
Cite this paper: Abrehet Fisseha, Effect of Redox Agents and Thermo Molding on the Network Formation and Stability of Wheat Gluten Bio-Plastics, International Journal of Food Science and Nutrition Engineering, Vol. 5 No. 5, 2015, pp. 203-208. doi: 10.5923/j.food.20150505.04.
Article Outline
1. Introduction
- Wheat composed of non-gluten proteins (15-20%) and gluten proteins (80-85%). The non-gluten proteins are monomeric metabolic/structural role with a molecular weight (MW) lower than gluten [37, 35]. The gluten proteins of wheat are further fractioned to gliadin, glutenin and an un-extractable fraction according to the Osborne fractionations [7, 35]. Gliadins and glutenins are usually found in more or less equal amounts in wheat [2, 13]. Gluten is less soluble in water or dilutes salt solution and gliadins are soluble in aqueous alcohols, which is not in the case for the glutenin (7, 35). Cysteine is a minor amino acid but extremely important for the structure and functionality of gluten [37]. Alterations of cross-links (disulfide, isopeptide, dehydroprotein, cross-links from Maillard reaction and tyrosine) are important during processing as it changes the chemical, functional and nutritional properties [12]. Disulfide bonds are formed by oxidative coupling of two cysteine residues that are adjacent within a protein matrix in the presences of oxidant which accepts the hydrogen atoms from the thiol groups is important for the stability of conformation [20, 35, 12, 29]. Heating increases the molecular weight of gluten aggregates and decreases protein extractability [20]. The effect of heating on the protein extractability depends on the gluten moisture content [37]. Hydrostatic pressure decreases gluten extractability, which is more pronounced at high temperatures [18, 1]. The glutenins are polymerized at lower temperatures than gliadins [20, 31].The formation or cleavage of disulfide bonds can be stimulated by the application of redox agents. The higher protein extractability after hydrothermal treatment of gluten in the presence of oxidants elucidates decreased cross-linking [28, 19, 2, 12, 35, 10, 32]. In contrast, the presence of dithiothreitol (DTT) resulted in lower protein extractability after hydrothermal treatment as of the reducing agent facilitate the gliadin-glutenin cross-linking during heating, while oxidants hinder gluten polymerization due to decreased levels of free SH groups and less flexibility of the glutenin chains. The conformational changes of gliadin-glutenin cross-linking model with heating was postulated [22, 20, 15]. Reducing agents lead to depolymerization of glutenin polymers before heating with an increase in the level of free SH groups. In contrast, oxidants decrease the level of free SH groups. The SDS extractability of proteins is a measure for the degree of polymerization; the lower the extractability the higher the degree of polymerization [11, 9, 27, 24, 6]. Process conditions and presence of additives influence the SDS protein extractability [21, 32, 8, 11, 39, 16, 6]. Plant proteins are hygroscopic in nature and bind water through hydrogen bridges. The water barrier properties and the water absorption capacity of gluten bio-plastic can be modified by thermal and chemical treatment [4, 33, 5, 8, 11, 9, 39]. Due to the pollution caused by synthetic polymers and the finiteness of petroleum resources, there has been a growing demand for polymers which are environment friendly and biodegradable, provide nutritional value and suitable gas barrier properties [39, 32, 3]. For this reason; bio based plastic are important to minimize the cause of synthetic polymers and recourses. The water barrier properties of wheat gluten films are very poor, but they have remarkable gas barrier properties dependent on the composition and process condition [21, 26, 26, 17, 23, 14].
2. Materials and Methods
- Wheat gluten from Syral (Aalst, Belgium) was fractionated based on their solubility difference in aqueous alcohols (70% ethanol) with continuous drying. The fractions were mixed at different mixing ratio and the moisture content was adjusted to 7%. The mixing ratio were gliadin-rich (100GLI), 75% 100GLI and 25% 0GLI (75GLI), 50% 100GLI and 50% 0GLI (50GLI), 25% 100GLI and 75% 0GLI (25GLI) and glutenin rich (0GLI). All the samples were thermo molded in a Pinette Press Zenith 2 (Pinette Emidecau Industries, Chalon sur Saône, France) at 5 bars at 130 and 150°C during 5 and 25 min after conditioning for 2 days at 50% relative humidity and 20°C. The moisture content was determined according to AACC Approved method 44-15A. The protein content was determined (AOAC, 1995) with a conversion factor 5.7. Protein extractability was measured with size exclusion (SE-) high performance liquid chromatography (HPLC) based on a difference in hydrodynamic volume. The free sulfhydryl content was determined with Sodium phosphate and Tris - HCl buffer calorimetrically after reacting with 5.5’-dithio-bis(2-nitrobenzoic acid) (DTNB). Amino acids including lanthionine and lysinoalanine were liberated by acid hydrolysis and separated by high-performance anion-exchange chromatography with integrated pulsed amperometric detection (HPAEC-IPAD) by the method of Rombouts et.al., (2009) [28]. Water absorption capacity of gluten plate (3 cm long, 1 cm wide and 0.1 cm thick) was determined by submerging in water containing 20 mg/l sodium azide for 72 hours.
3. Results and Discussion
3.1. Extraction Yield
- After extraction with aqueous alcohol solution 38% glutenin rich (0GLI) and 42% gliadin rich (100GLI) were obtained. The remaining 20% was starch and non-gluten components washed away during extraction. The protein content of gluten, 0GLI and 100GLI were respectively 76%, 79% and 86%.
3.2. Protein Extractability
- As shown in fig 1 the protein extractability profile expressed as SDSEP of gluten, 0GLI and 100 GLI in sodium dodecyl sulfate (SDS) in the SEHPLC; the gliadin peak (left side) and glutenin peak (right side) in the chromatograph for all samples. The gluten, gliadin rich and glutenin rich have both gliadin and glutenin peak. The protein extractability profile of gluten, 0GLI and 100GLI; the gliadin peak of 100GLI is higher than the gliadin peak of gluten. The gliadin peak of glutenin rich fraction shows smaller peak as neither the gliadin nor the glutenin are pure fractions. Indeed, the gliadins are soluble in the SDS containing buffer, but not all glutenins. The extractability of gluten and gluten fraction molded at 130 and 150°C during 5 and 25 min with SDS containing buffer were significantly affected Figure 2. During thermo-molding protein extractability decreases as polymerisation reaction occurs due to the presence of disulfide and non-disulfide cross-links. The extractability decreases with the molding time and temperature and with glutenin. The larger decrease extractable glutenin content explains the susceptible property to thermomolding than gliadins. Probably, the formation of intermolecular disulfide bonds between an unextractable glutenin and an extractable glutenin is enough to make the entire aggregate. In 100GLI, no unextractable glutenins are present. In order to obtain unextractable aggregates the extractable glutenins have to react with each other or with the gliadins due to the cross linking of the disulfide bond.
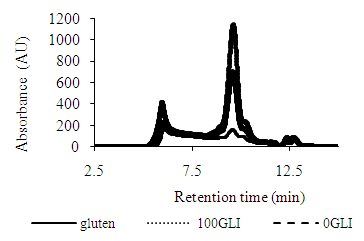 | Figure 1. SE-HPLC chromatogram of commercial gluten, 0GLI and 100GLI, extracted with SDS containing buffer |
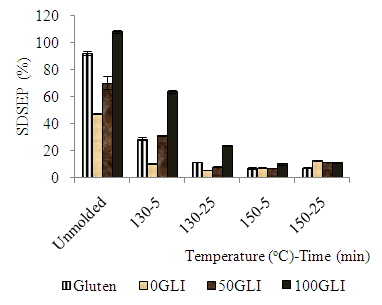 | Figure 2. The Protein extractability (SDSEP %) content of gluten and gluten fractions before and after molding |
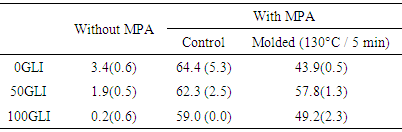
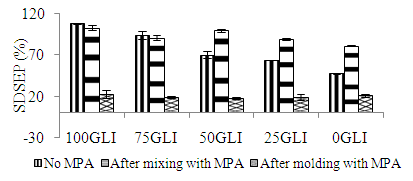 | Figure 3. The SDSEP of gluten fractions with and without MPA after molding at 130°C for 5 min |
3.3. Free SH Content
- Free SH groups of gluten and gluten fractions play an important role in the network formation during heat treatment (Table 1 and 2). The free SH content was measured equivalent with sodium phosphate and Tris/HCl buffer. An increase in the free SH content with increasing glutenin content was observed explained the presence of free SH contents in glutenins but not in gliadins. However, the free SH content of 50GLI seemed lower compared to that of gluten a likely explanation for the occurrence of oxidation reactions during the extraction. With increasing molding temperature and time the free SH content increased due to β-elimination reactions results in the cleavage of cystine residues with the formation of cysteine (and thus free SH), DHA and atomic sulfur [10] (fig 4). The free SH content after molding at 150°C for 25 min is the same for all fractions, while the free SH content before molding depends on the gliadin-glutenin ratio. The stronger increase in free SH content for gliadin-rich fractions indicates the more β-elimination reactions occurs than glutenin-rich fractions. In the presence of MPA the free SH content of gluten fractions has increased as the MPA composed of small amount of free SH group. After thermo-molding the free SH content was reduced as oxidation has occurred during overnight mixing, removal of the solvent and/or thermal treatment.
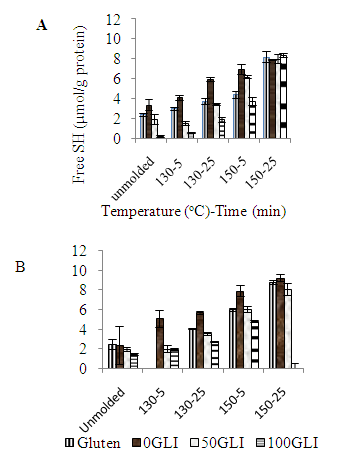 | Figure 4. The free SH content of gluten and gluten fractions before and after molding. (A) Method with sodium phosphate buffer. (B) Method with Tris/HCl buffer |
|
|
3.4. Amino Acid Analysis
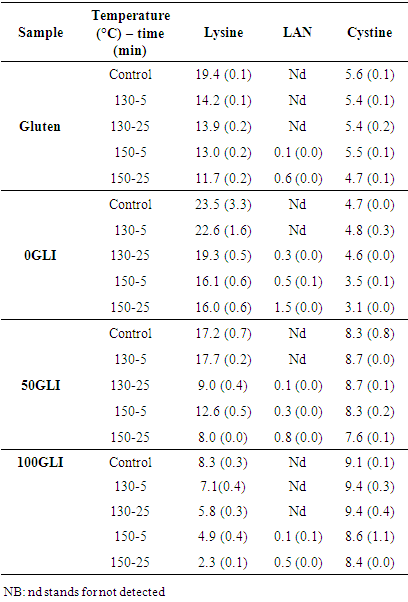
- Three amino acids which were significantly affected by molding: cystine, lysine and LAN presented below in (table 3). The decrease in cystine with increasing molding temperature and time was observed but it was only significant after thermomolding at 150°C during 25 min. As a result increase in free SH content after molding and a decrease of the cystine content was observed. No LAN was detected in unmolded gluten and gluten molded at 130°C during 5min. The low lanthionine (LAN) content was detected in gluten molded at 130°C during 25 min and the LAN content increased with increasing molding temperature and time. An increase in LAN content with increasing molding condition was detected for all gluten fractions but higher increment was with increasing glutenin content due to β-elimination reactions and formation of intermolecular disulfide bonds. The lysine content decreased with increasing molding temperature and time was due to the formation of lysinoalanine (LAL) when reacting with dehydroalanine (DHA) and consumed by maillard reaction.
|
3.5. Water Absorption Capacity
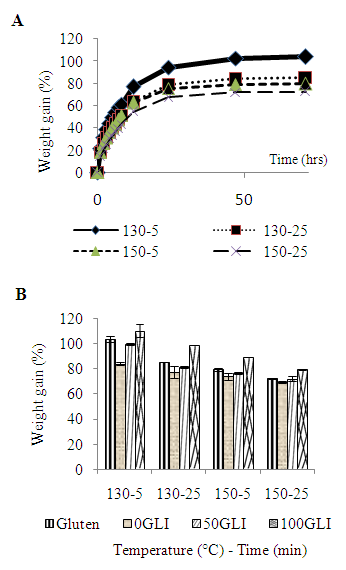
- The water absorption capacity was determined for two reasons (1) Material property and (2) To generate information on the degree of cross linking (the lower water absorption is consistent with an increased degree of cross-linking). The water absorption for gluten molded at different molding conditions is shown in (fig 5). Most of the water was absorbed during the first 24 hours of submersion. After 48 hours constant water was absorbed. The water absorption of gluten molded at 130°C during 5 min was more than 100%. For practical use of gluten bioplastic water absorption needs to be reduced thought with increased molding condition the water absorption capacity was reduced due to an increase degree of cross-linking and decreases protein extractability. However, the decrease in water absorption was much stronger for the gliadin-rich samples than for the glutenin-rich samples due to the maximal degree of cross-linking.
4. Conclusions
- Gluten is annually renewable and perfectly biodegradable. However, the current gluten based materials are still outperformed by their synthetic counterparts. In this regards, it is important to understand the gluten network formation during the production of bio plastic. The gliadin and glutenin mix were thermo molded at different molding condition (130 and 150°C during 5 and 25 min) with and without redox agents. The protein extractability, free SH content and water absorption capacity of the gluten, gliadin and glutenin were positively affected with the redox agents and molding condition. The increment in protein extractability and decrement of free SH with gliadin content were an indication of the gliadin are lesser susceptible to thermo molding than glutenin. Thought with increased molding condition protein extractability of gliadin was reduced due the occurrence of disulfide bond thought the free SH content were increased due to polymerization reactions. As a result the network formation of the molded gluten and its fractions were positively affected and the water absorption capacity of the bio-plastic plates reduced with the molding condition, redox agents and composition due to the consistent cross linking increment.
ACKNOWLEDGEMENTS
- The author thanks all who have put their efforts from the start to end of the research experiments, expressly KULeuven, Laboratory of Food Chemistry and Bio Chemistry, VLOR –UOS and Hawassa University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML