-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2015; 5(4): 169-175
doi:10.5923/j.food.20150504.02
Advances in Antimicrobial Food Packaging with Nanotechnology and Natural Antimicrobials
Sanjaysinh Makwana1, Ruplal Choudhary1, Punit Kohli2
1Department of Plant, Soil and Agricultural Systems, Southern Illinois University Carbondale IL, USA
2Department of Chemistry and Biochemistry, Southern Illinois University Carbondale IL, USA
Correspondence to: Ruplal Choudhary, Department of Plant, Soil and Agricultural Systems, Southern Illinois University Carbondale IL, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Antimicrobial Packaging is an area of emerging interest and it is rapidly advancing with the application of nanotechnology and natural antimicrobials. This review describes recent research and developments in antimicrobial food packaging by application of natural antimicrobials and nanomaterials in food packaging materials. In addition to the development made in antimicrobial packaging by nanotechnology, global market and regulation aspects are also discussed. Even though rapid research and development are made in this area, successful application is limited. Besides the research on application of nanotechnology to food contact material there is need for toxicology studies of different types of nanomaterials.
Keywords: Nanotechnology, Nanoparticle, Liposome, Antimicrobial food packaging, Natural antimicrobials
Cite this paper: Sanjaysinh Makwana, Ruplal Choudhary, Punit Kohli, Advances in Antimicrobial Food Packaging with Nanotechnology and Natural Antimicrobials, International Journal of Food Science and Nutrition Engineering, Vol. 5 No. 4, 2015, pp. 169-175. doi: 10.5923/j.food.20150504.02.
Article Outline
1. Introduction
- The natural deterioration of fresh or processed foods is caused by their interaction with a variety of elements including water or gases, by contamination with bacteria, yeast, or fungi, or by infestation by insects and rodents. Also, food is a first-rate medium through which human pathogens can spread easily. Food packaging performs four basic functions: protection, containment, information, and ease of use. Of these functions, protection of food from invasion of undesirable pathogenic or spoilage microorganisms is considered the most important. Advancement in food packaging is closely related with advancement in material science and processing technologies. With the advancement in science and technology, various packaging technologies have been developed such as metal can, can with aluminum alloys, heat shrinkable polyvinyl chloride, controlled atmosphere packaging, modified atmosphere packaging, vacuum packaging, active packaging and antimicrobial packaging. (Cutter, 2002). One of the approaches which can play an important role to ensure microbial safety of the packaged food is the antimicrobial packaging. The advancement of nanotechnology may enable the antimicrobial packaging as a successful application leading to regular use in food packaging. Nanotechnology can play an important role in the development of food packaging. One of the applications includes incorporation of nanocomposite into the food package to improve the barrier property (Restuccia et al., 2010). There has been over $17.8 billion investment across the globe in 2010 for research and development in nanotechnology (Sargent Jr., 2013) and it is estimated that by 2020 it will grow to $3 trillion (Roco, 2011). In the area of food nanotechnology research and development, the most active area is packaging. Interesting applications of nanotechnology in food packaging include development of intelligent packaging and active packaging, including antimicrobial packaging, as well as nanotechnology for barrier and mechanical reinforcement (iRAP Inc., 2011; Duncan, 2011).
2. Antimicrobial Packaging
- Active packaging is an innovative food packaging concept that helps to meet new trends in the area of food distribution. Active packaging includes additives that absorb undesirable component from food such as oxygen, ethylene, moisture and odor to enhance the quality and shelf life of the food (Prasad and Kochhar, 2014). Active packaging also includes the release of antimicrobials and preservative into the food to enhance microbial quality and safety of the food. Different types of active packaging are controlled atmosphere packaging, modified atmosphere packaging, and antimicrobial packaging. Among these, the antimicrobial packaging research is getting more attention of researchers because of its critical role in improving microbial safety and extending shelf life of food products. The techniques of antimicrobial research involve incorporating or coating packaging materials with antimicrobial agents for slow release in to the packaged food system to inhibit the growth of microorganisms to maintain food quality and safety (Mauriello et al., 2005).The slow release of antimicrobials in to the food system by their incorporation in to the food packaging is more efficient than direct addition in to the food because it may react with other food components and results in loss of its activity. For example, the antibacterial activity of silver ion was strongly reduced by the protein rich food (Ilg1 & Kreyenschmidt, 2011) because it has high binding potential to the cysteine, methionine, lysine and arginine (Gruen, 1975). Therefore use of antimicrobial packaging film may be more efficient which also ensures residual activity over longer period of time during transportation and storage.
3. Natural Antimicrobials
- In recent years because of government regulations and health concerns there has been rising attention towards the use of natural antimicrobial for food safety application. For example Nisin, different plant extracts i.e. cinnamaldehyde, allyl isothiocynate, oregano and carvacrol are gaining more and more attention for food safety application. In this section, we have discussed some of the prominent antimicrobials being researched in antimicrobial packaging.
3.1. Nisin
- Nisin is a ribosomally synthesized peptide that has broad-spectrum antibacterial activity. It is produced as a fermentation product of a food-grade bacterium. Nisin is the most frequently studied antimicrobial in active packaging system and it is the only bacteriocin that has been allowed to use as preservative in food. Nisin as an antimicrobial agent in packaging shows 3 to 7 log reduction in most of the studies (Joerger, 2007). Nisin in combination with ally isothiocyanate completely inhibited the Salmonella to an undetectable level in liquid egg albumen (<10 CFU/ml) (Jin and Gurtler, 2011), and polylactic acid coating with nisin also completely inactivate Listeria monocytogene in skim milk (Jin, 2010). These studies were carried out by coating of these antimicrobials on inner surface of glass jar and exposure of microbes in liquid food under the shaking condition. Nisin coated polyethylene films also exhibited considerable antibacterial activity against Listeria monocytogenes in a food system other than liquid food such as tofu (Joerger, 2007, Cutter 2002).
3.2. Chitosan
- Chitosan is the second most plentiful polysaccharide found in nature after cellulose. It is a linear polysaccharide consisting of (1,4)-linked 2- amino-deoxy-β-D-glucan, is a deacetylated derivative of chitin. Chitosan with molecular weight 10 kD to 100 kD has shown most antibacterial effect (Dutta et al., 2009), however with different susceptibility to the Gram positive and Gram negative bacteria (Joerger et al., 2009). In addition to being an effective antimicrobial, chitosan is nontoxic, biodegradable, bio functional and biocompatible. Various studies have been carried out on the antimicrobial film incorporated with chitosan. Ethylene co-polymer film with chitosan attachment showed 5 log reduction of E.coli and 2-3 log reduction of L. monocytogenes in 24 hr in phosphate buffer medium but had 2 log less activity in packaged food (Joerger et al., 2009). Chitosan also shows good antibacterial activity in combination with other antimicrobials. Incorporating chitosan with lauric acid into starch film showed more effective antimicrobial ability against B. subtilis and E. coli than the chitosan alone (Salleh et al., 2007). Edible film of Konjac glucomannan casted with chitosan and nisin also showed synergistic antimicrobial effect (Li et al., 2006).
3.3. Acids and Their Salts
- These groups of antimicrobials, such as sorbic acid, potassium sorbate, acetic acid, citric acid, and lactic acid have also been studied in respect to the antimicrobial food packaging application. Most acid containing films were reported to reduce target organism, such as mesophilic and psychrophilic bacteria by 5 log unit or less (Joerger, 2007). Study carried out by Jin et al. (2011) showed that poly lactic acid coating with 500 µl ally isothiocyanate (AIT) completely inactivated 3 and 7 log CFU/ml of Salmonella after 7 and 21 days of storage, respectively. When 200 µl of AIT in combination with 250 mg nisin was used, Salmonella population was reduced to an undetectable level (<10 CFU/ml) after 21 days of storage in liquid egg white medium (Jin et al., 2011).
4. Promising Natural Antimicrobials
- Bio-preservatives are becoming increasingly important for food safety application because they are safe for human consumption. Bio-preservatives are potential antimicrobials that include a group of antimicrobials that occur in nature or have been isolated from plant or animal sources. Few examples of bio-preservatives are plant and spice essential oils, lysozyme, fruit peel, seed and leave extracts. Edible food packaging films with List of natural antimicrobials from spices and other plants are increasing day by day. A few prominent potential natural antimicrobials have been discussed in this section.
4.1. Plant and Spice Essential Oils
- Traditionally, essential oils have been used widely in perfumery, aromatherapy, cosmetics, and for flavoring food and drink, and to a lesser extent in medicine and household cleaning products (Nychas, 1995). The essential oils are usually aromatics extracted from the plant based materials and they possess antiviral, antibacterial, antimycotic, antitoxigenic, antiparasitic and insecticidal properties. Their use as food grade additives is allowed in the USA, and therefore the antimicrobial activity of these essential oils has caught attention of food scientists working on food safety r (Bevilacqua et al., 2010). Citrus extract comprises of antioxidants and flavonoids, such as rutin, naringin, quercetin as well as phenolic compounds. Common spoilage micorflora of fruit juice S. bayanus, R. bacarum, P. membranifaciens, B. coagulans, L. brevis and L. plantarum are reported to be very susceptible to the citrus extract and minimum inhibition concentration was 2 ppm, 3 ppm, 5 ppm, 20 ppm, 40 ppm and >40 ppm respectively (Bevilacqua et al., 2010). The antimicrobial properties of essential oils of spices have been recognized since ancient time, and traditionally it is used as condiments and to enhance taste or smell of certain foods. They consist of simple alcohol, aldehyde, ketones, phenols, esters and organic acids. The minimum inhibition concentration of selected spice essential oil against E.coli strains were studied by Santurio et al. (2011). Essential oil of oregano was most effective antimicrobial against E.coli with MIC value of 750.1 µg/ml. The MIC value of essential oils cinnamomum zeylanicum (cinnamon), lippia graveolens (Mexican oregano), thymus vulgaris (thyme) and origanum vulgare (oregano) against E.coli was 3098.5 µg /ml, 1508.7 µg/ml, 1941.5 µg /ml, 834.1 µg /ml respectively (Santurio et al., 2011). In one study, volatile components from essential oils of mustard, cinnamon, garlic acid and clove were added to filter paper to inhibit spoilage fungi such as Aspergillus flavus, Endomyces fibuliger, the common spoilage fungi of bread. The mustard essential oil showed the strongest antimicrobial effect (Nielsen and Rios, 2000).
4.2. Pomogranate Peel Extract
- Pomogranate is a tropical fruit that has been traditionally used in Indian sub-continent as a common home remedy for stomach upset, fever, cough and cold, and diarrhea. The edible part of this fruit contains saccharides, polyphenols, and minerals. The predominant phytochemicals of pomogranate are polyphenols i.e. gallic acid, catechin, rutin, anthocyanidins (Gil et al., 2000). The polyphenols extracted from pomogranate peel has shown broad spectrum antimicrobial activity against bacteria and fungi. The antifungal activity of methanolic extract of pomogranate was reported to be highest against A. niger followed by P. citrinum, R. oryzae, T. reesei and M. indicus. The antibacterial activity of methanolic extract of pomogranate peel was highest against S. aureus with the zone of inhibition of 10-25 mm (Dahham et al., 2010).
4.3. Grape Seed Extract
- Grape skin and seed are rich source of polyphenolic compounds mainly consisting of catechin, epicatechin, gallic acid, polymeric and oligopolymeric procyanidins, and both were found to be effective antimicrobial against Gram positive and Gram negative bacteria (Bayder et al., 2004). Compared to grape skin extract, grape seed extract possesses better antimicrobial activity. Grape seed extract contains naringin, ascorbic acid, hesperidin, and various organic acids such as citric acid. It exhibits a wide range of antimicrobial activity. Its water soluble fraction contains triclosan and methyl-p-hydroxybenzote. The antimicrobial activity of grape seed extract immobilized on packaging films inhibited growth of aerobic bacteria coliforms on fresh produce when contact packaging was used (Han, 2005). The antimicrobial activity of grape seed extract was highest against S. aureus, E. faecalis, and K. pneumonia followed by E.coli and P. aeroginosa with diameter of inhibition zone against these microorganisms was reported to be 16.2, 16.2, 16, 15 and 14.2 mm respectively (Nirmala et al., 2011).
4.4. Mexican Tarragon
- Mexican tarragon has been used since the Aztec time for medical purposes and has currently become a popular herb. It is used as tea in Mexico to reduce the stomachache and to calm mental agitation. It is popular in southern states of United States since it is well known substitute for European tarragon. The methanolic extracts dihydroxylated coumarins were found to be most effective antimicrobials on Gram negative and Gram positive bacteria. The dimethoxy compounds possess strong antifungal activity especially on T. mentagrophytes and R. solani, causing 100 % inhibition at 125 and 250 µg/ml respectively (Ceaspedes et al., 2006).
4.5. Olive Extract
- Traditionally olive leaf has been used as a home remedy against fever and infections. Most of the past research had focused on antibacterial properties of olive fruit, however, recently more and more studies are finding antimicrobial activities of olive leaves. Olive fruit extracts containing phenolic compounds have significant antibacterial activity against Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus. A glycoside oleuropein from olive fruit was reported to inhibit the sporulation of Bacillus cereus. In case of olive leaf, its water extracts showed significant antibacterial activity against E.coli, S. aureus, K. pneumonia and Pseudomonas aeruginosa (Markin et al., 2003). The antibacterial activity of leaf extract were studied by exposing cell culture to the water extract of olive leaf. At 30 % concentration of olive leaf in medium it completely inhibited the Candida albicans (Markin et al., 2003).
4.6. Wasabi Extract
- Volatile allyl isothiocyanate (AIT) which is reported as active agent in antimicrobial packaging is the main active antimicrobial component of wasabi extract. The minimum inhibition concentration (MIC) of AIT against Yersinia enterocolitica, and L. monocytogenes was in the range of 14 to 145 ppm at 10-40 C (Han J H, 2005). At higher relative humidity, AIT exhibited stronger antimicrobial activity.
5. Nanotechnology Driven Antimicrobial Packaging
- Nanotechnology involves the characterization, production and use of material that have dimension of about 1-100 nm. When particle size is reduced to this dimension the resulting particle exhibits different physical and chemical properties. In the food industry there is a scope of nanotechnology in almost every sector (Duncan, 2011). The most active area of food nanotechnology research and development is food packaging. The global market of food and beverage packaging using nanotechnology grew from $150 million in 2002 to $860 million in 2004 (Buzby, 2010). Recent examples of nanotechnology in food packaging include addition of nanoparticles and nanoclays in to the food packaging to increase its barrier property and also improving antibacterial property of packaging materials for food safety and preservation (Bouwmeester et al., 2009).
5.1. Inorganic Nanoparticles in Food Packaging
- There are two types of antimicrobial used in food industry, organic and inorganic. Incorporation of inorganic nanoparticles in food packaging serves various objectives, such as improves physical performance, durability, barrier properties and biodegradation. Coating of certain nanoparticles on food packaging film or material serves the objectives of food preservation and safety as these nanoparticles exhibits potential antibacterial activity against food spoilage microorganism and food borne pathogens. (Bradley et al., 2011). Nanoparticles such as nanoclays are incorporated into plastic beer bottles to increase strength, make them more shatterproof, and extend shelf life by acting as a barrier to keep oxygen outside the bottle and carbon dioxide inside. In the following sections we review some selected inorganic nanoparticles being used in packaging materials to improve safety and quality of food.
5.1.1. Zinc Oxide Nanoparticle
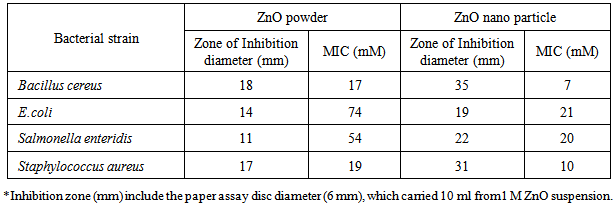
- Inorganic nanoparticles such as ZnO have received significant attention in recent years because of their stability under intensive processing conditions and antibacterial activity. Zinc oxide nanoparticles are already integrated into a variety of medical and skin coatings because of their antimicrobial and/or antifungal properties (Tayel et al., 2010).Table 1 shows antimicrobial activity of ZnO powder and nanoparticles against food borne pathogens. The ZnO nanoparticles showed better antibacterial activity than non-nano powder against all the tested bacteria (Li et al., 2009). The development of ZnO-coated food packaging film has drawn considerable attention of the food packaging industry.
|
5.1.2. Silver Nanoparticle
- As a result of increase in resistance of bacteria to the antibiotics, studies have been done on antimicrobial in the form of nanoparticles as effective bactericidal materials. Highly reactive metal oxide nanoparticles exhibits excellent biocidal property against Gram positive and Gram negative bacteria. Silver nanoparticles has long been known for its antibacterial properties and it is being incorporated in to textiles, food packaging, coating of medical devices, dental resin components and other material where antimicrobial properties are desirable (Morones et al., 2005). Silver nanoparticles in the range of 1-10 nm attach to the surface of cell membrane and alter the cell wall permeability and respiration, and after penetrating inside the bacteria it causes damage to the DNA (Morones et al., 2005). It has been also reported that antibacterial activity of such as penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin increased in the presence of silver nanoparticles against Staphylococcus aureus and Escherichia coli (Shahverdi et al., 2007). The food application of silver nanoparticles includes antimicrobial packaging for food safety and preservation. In one food packaging study, biosynthesized silver nanoparticles incorporated into sodium alginate films showed significant antibacterial effect against E.coli, and S.aureus (Fayaz et al., 2009). In Another study silver nanoparticles were deposited on multilayered linear low density polyethylene (LLDPE) by laminating, casting and spraying. The antimicrobial activity of such films showed 70% reduction of fungus A. niger in 3 h at 24°C (Sánchez-Valdes et al., 2009).
5.1.3. Copper Nanoparticle
- The germicidal and biocidal properties of copper containing substance are well known but there have been limited studies carried out on copper nanoparticles. From last century, copper or copper oxide particles added into organic matrixes as antifouling coatings by the paint industry, mainly for maritime applications. Copper nanoparticles were shown to inhibit microbial growth of Saccharomyces cerevisiae, Escherichia coli, Staphylococcus Aureus and Lysteria monocytogenes on a polymer composite after 4 hr of exposure in diluted liquid broth media (Cioffi et al., 2005). Sheikh et al. (2011) also demonstrated good antibacterial effect of copper nanoparticles against E.coli and B. subtilis in polyurethane nanofibers containing copper nanoparticles.
5.1.4. Titanium Oxide Nanoparticles
- Titanium dioxide is a naturally occurring oxide of titanium. As the bulk form titanium oxide often contains a nano-sized fraction, nano- Titanium oxide has been in use for many years without being known as nano. It is a photocatalyst and widely utilized as self-disinfecting coating of surfaces (Fujishima et al., 2000). It is non-toxic and has been approved by the FDA for use in food, drugs, cosmetics and food contact material not more than 1% by weight. Titanium oxide has also been widely studied for its antibacterial activity against wide spectrum of microorganisms. The antibacterial activity of oriented-polypropylene (OPP) film coated by titanium oxide powder was studied by Chawengkijwanich et al. (2008). This film showed 3 log reduction of E.coli cells after 180 min of illumination by two 20 W black-light bulbs (wavelength of 300–400 nm), compared to uncoated OPP film with black-light illumination decreased E. coli by 1 log CFU/ml.
5.2. Nanoscale Liposome in Food and Food Packaging
- Liposomes are bilayers of polar lipids enclosing an aqueous core. Food application of liposomes includes nutrient encapsulation and delivery, encapsulation of functional component such as proteins and enzyme, antimicrobials and flavors to prevent from degradation from adverse conditions in food system. Liposomal encapsulation of spice and herb extract which are natural antimicrobials, offers several benefits such as both non-polar and polar compounds can be encapsulated and thus protected from degradation. Limited studies reported application of liposomal encapsulated natural antimicrobials in food packaging. Mekkerdchoo et al. (2009) reported the antimicrobial activity of pectin film containing liposome encapsulated antimicrobial extract i.e. clove oil, garlic oil and pomogranate extract. The results showed that antimicrobial activity of the pectin film containing encapsulated antimicrobial was more compared to the pectin film containing antimicrobial extract without encapsulation. Clove oil had significantly larger inhibition zone (4.75 cm) against Lactobacillus sake (Mekkerdchoo et al., 2009). It has been also reported that antimicrobial and antioxidant activity of Myrtus communis extracts improved after encapsulation in to liposome (Gortzi et al., 2008). The encapsulation of these extracts in liposome produced 25% more antioxidant activity than the same extract in pure form. Addition of liposome encapsulated protease in to cheese curd reduced the time and cost to ripen some hard variety of cheese (Taylor, 2005). Recent study on liposome encapsulation of cinnamaldehyde and curcumin showed significant enhancement of antibacterial activities against E.coli W1485 after encapsulation (Makwana, 2013; Makwana et al., 2014).
6. Government Regulation of Nanotechnology in Food
- One important concern about the nanotechnology is the public health and safety because of migration and toxicity of active nanoparticles. In the United States active and intelligent packaging is not subject to any specific regulatory concern. There is limited scientific evidence of the extent to which nanoparticles may enter and possibly accumulate t in the body (Chau et al., 2007). It was reported in March 2006 that more than 70 people were hospitalized after using a bathroom cleaner manufactured using nanotechnology. Some studies revealed that long term exposure to an environment containing carbon nanoparticles would cause vascular disease. According to guidelines by Organization for Economic Co-operation and development (OECD), the key physical-chemical parameters to take in to account for testing specific manufactured nanomaterials for human health and environmental safety are: agglomeration and/or aggregation, water solubility, crystalline phase, dustiness, crystallite size, particle size distribution - dry and in relevant media, specific surface area, zeta potential (surface charge) , surface chemistry (where appropriate), photocatalytic activity, pour density, porosity, octanol-water partition coefficient where relevant, redox potential, radical formation potential, and other relevant material characterization information (OECD, 2010). So far the food and drug administration of USA (FDA) is not aware of any food substances intensely manufactured on the nanometer scale for which safety data is available for its determination to use as a GRAS food substance (FDA, 2014). More data is required for the regulatory agencies like FDA and OECD to implement policy on safe use of nanomaterials in contact with food.
7. Conclusions
- Antimicrobial food packaging play an important role in reducing the risk of post processing pathogen contamination, as well as extending the shelf life of food. It is gaining global interest across the food industry and research community as it provides quality and safe food. Currently the development and commercialization of antimicrobial food packaging is limited because of availability of safe antimicrobials, cost involved in use of natural antimicrobials and regulatory concerns. There is a great need to exploit nanotechnology to develop cheap, practically feasible antimicrobial coating and packaging that can withstand the environment present in the food system without affecting the food quality and sensory attributes. More research is required in safety assessment of engineered nanoparticles being targeted to use directly into food or in food-contact surfaces such as food processing and handling equipment or food packaging materials so that enough data is available for the regulatory agencies to draft universal safety policy on use of nanomaterials intended to be in food contact.
ACKNOWLEDGMENTS
- This research was partially supported by the US-Israel Binational Agricultural Research and Development (BARD) Grants US-4471-11F and US-4680-13C, and NIH GM-106364.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML