-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2015; 5(3): 101-114
doi:10.5923/j.food.20150503.01
An Evaluation Study of the Cultivation of Spirulina in the Area of Sambirano, Madagascar
Zafilaza Armand1, Andriantsimahavandy Abel1, Ramamonjisoa Daniel Joseph1, Andrianainarivelo Mahandrimanana2
1University of Antananarivo Madagascar, Faculty of Sciences, Department of Fundamental and Applied Biochemistry
2University of Antananarivo Madagascar, Faculty of Sciences, Department of Inorganic Chemistry and Physics Chemistry
Correspondence to: Andrianainarivelo Mahandrimanana, University of Antananarivo Madagascar, Faculty of Sciences, Department of Inorganic Chemistry and Physics Chemistry.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The area of Sambirano is very favourable to the cultivation of Spirulina. The climates, salinity, the pH, the structure of the ground allow the cultivation of Spirulina. The results show that the rates of the components are not differing in the South from Madagascar. Therefore, to ensure and stabilize the components, it is necessary to set up the method HACCP and the strict method of conservation.
Keywords: Spirulina, Physico, Chemical Analysis, Microbiological Study
Cite this paper: Zafilaza Armand, Andriantsimahavandy Abel, Ramamonjisoa Daniel Joseph, Andrianainarivelo Mahandrimanana, An Evaluation Study of the Cultivation of Spirulina in the Area of Sambirano, Madagascar, International Journal of Food Science and Nutrition Engineering, Vol. 5 No. 3, 2015, pp. 101-114. doi: 10.5923/j.food.20150503.01.
Article Outline
1. Introduction
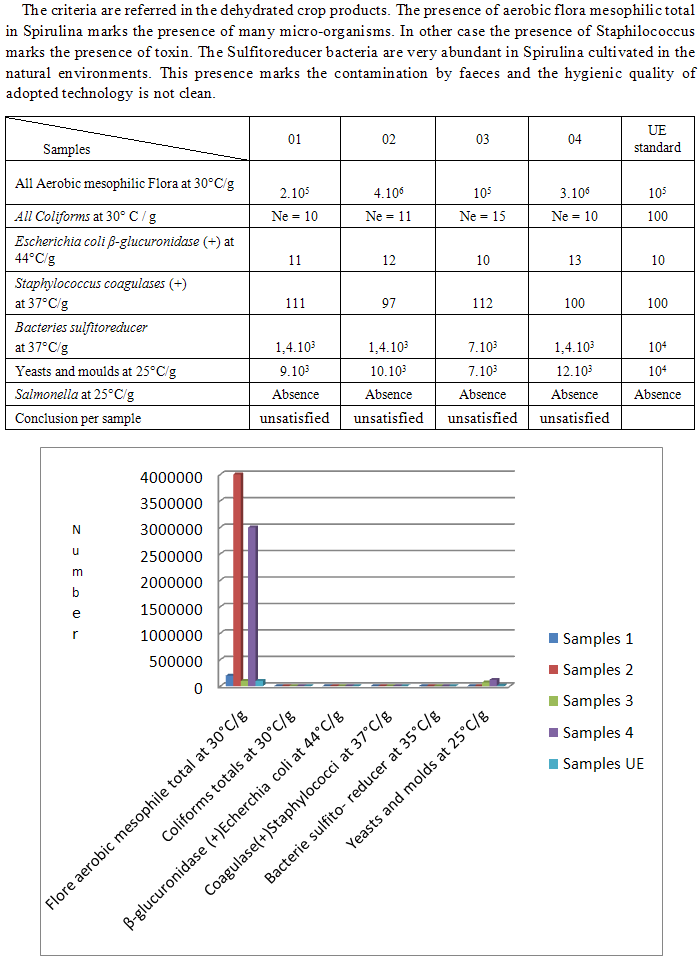
- Sambirano is in the northewest, District of Ambanja in the region DI.A.N.A. The climate is rainy. The pH, the salinity and the turbidity are favorable to the cultivation. Spirulina is easy to cultivate but requires adequate parameters to develop. A potential problem of Spirulina production in open system is the hazard of water contamination with pathogenic organisms. Handling of the product during processing, harvesting and drying, can also result in microbial contamination. The final microbial load of the product will therefore depend on how carefully the culture and product are handled at the various stages of production [7]. Only good manufacturing practices and direct analysis of microbial flora as well as concentration in each lot of product can guarantee the safety of the product. The final product should meet microbiological standards set by the various national and international standards [7, 14].The production of high quality Spirulina therefore requires the use of high-grade nutrients and routine analysis of heavy metals in the culture medium and the product. This is particularly important in situations where food-grade Spirulina is produced from open-ponds or natural lakes [7, 23, 41].Our study is based in the two more or less different cultivations. One is cultivated in the natural environment (Mn) and the other in the improved natural environment (MnA). That’s why the main aim of this work is physicochemical analysis of the two samples and follow-up the microbiological analysis. This study is divided into three parties. Firstly, bibliographical study. Second is devoted to the methods of analysis followed by results of the parameters physicochemical and microbiological before and after the conservation. Third, the discussion and finally the conclusion.
2. Material and Methods
2.1. Description of the Study Area
- Sambirano is an area in the Northwest of Madagascar, in the region of Diana. The name refers to the Sambirano Valley as well as the Sambirano River which runs from the foothills of the Tsaratanana Massif into the Ampasindava Bay where it joins with the Ramena River, south of Ambanja. Due to the proximity of the Tsaratanana mountain range and trade winds, a particular micro-climate occurs in the Sambirano region. During the rainy season, the river floods and deposits extremely fertile alluvia along its river banks, providing ideal conditions for many types of crops, especially cacao. The rainfall is approximately, 2,000 mm per year in the Sambirano area in the northwest. The temperatures at higher elevations are mainly moderate, between 15° and 25°C. There is a cool, dry season between July and September and a warmer wet season during the rest of the year.
2.2. Morphology and Taxonomy
- a) MorphologySpirulina is symbiotic, multicellular and filamentous blue-green microalgae with symbiotic bacteria that fix nitrogen from air. Spirulina can be rod- or disk-shaped. Their main photosynthetic pigment is phycocyanin, which is blue in colour. Spirulina are photosynthetic and therefore autotrophic. Spirulina reproduce by binary fission. The helical shape of the filaments (or trichomes) is characteristic of the genus and is maintained only in a liquid environment or culture medium. The presence of gas-filled vacuoles in the cells, together with the helical shape of the filaments, result in floating mats. The trichomes have a length of 50 to 500 μm and a width of 3 to 4 μm. Under light microscopy, the blue-green non-heterocystous filaments, composed of vegetative cells that undergo binary fission in a single plane, show easily visible transverse cross-walls.Filaments are solitary and free floating and display gliding motility. The trichomes, enveloped by a thin sheath, show more or less slightly pronounced constrictions at cross-walls and have apices either slightly or not at all attenuated [11, 16].Spirulina is characterized by its regularly coiled trichomes. Under some conditions of temperature and pressure, its helical filaments can convert to abnormal morphologies, such as irregularly curved and even linear shapes, that are considered as a permanent degeneration that could not be reversed. However, the linear filaments of Spirulina platensis could spontaneously revert to the helical form with the same morphology as the original filaments. The ultra-structural, physiological, and biochemical characteristics of linear filaments are different from those of the original filaments, whereas they are the same for the reverted and the original filaments [40].Spirulina is naturally found in tropical regions inhabiting alkaline lakes (pH 11) with high concentration of NaCl and bicarbonates [31].b) TaxonomyAccording to the classification in Bergey’s Manual of Determinative Bacteriology, Spirulina belongs to the oxygenic photosynthetic bacteria that cover the groups Cyanobacteria and Prochlorales, which are, by phylogeny, related to the sequence of the rRNA (ribosomal ribonucleic acid) sub-unit 16s. As a function of the sequence data of this sub-unit and the rRNA sub-unit 5s, these prokaryotes are classified within the Eubacteria group.
3. Qualitative Analysis
- The spirulina is cultivated in the North of Madagascar beside mangrove swamp called “Fasira”.The chemical analysis of Spirulina is done in two more or less different samples. One sample coming in the improved natural environment and the other comes in the natural environment. The two samples are analyzed by spectrophotometer u.v. or atomic absorption spectrophotometer [6, 8, 13, 15, 32].
4. Risk Evaluation Microbial Contamination
- a) Studied Samples– powder of Spirulina coming from the natural environment (Mn) – powder of Spirulina coming from the improved natural environment (MnA).b) Types of the studied microbes – aerobic flora mésophile total with 30°C– Coliforms totals with 30°C – Escherchia colib-glucuronidase (+) with 37°C– Staphilococca coagulases (+) with 37°C– bacteria sulfitoreducer with 37°C– yeasts and moulds with 25°C– Salmonella [3-5] c) Mother solutionOne takes 10g of Spirulina and to add 90g plugged peptoned water. The unit is to crush during 60s with the STOMACHER. After the solution mother is sudden a serie of decimal dilutions b) Way of calculating – in-depth sowing [2-5].
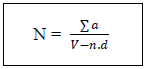
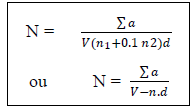 N: Number of coloniesThe sum of Ufc in two dilutionsV: Volume of inoculum ensemencedn1: number of limp of the 1st dilutionD: factor of dilution corresponds to the 1st dilutionn2: numbers of limp of the second dilutionN: number of limps– sowing on the surfaces [2-5] for the Staphilococcus coagulase (+)
N: Number of coloniesThe sum of Ufc in two dilutionsV: Volume of inoculum ensemencedn1: number of limp of the 1st dilutionD: factor of dilution corresponds to the 1st dilutionn2: numbers of limp of the second dilutionN: number of limps– sowing on the surfaces [2-5] for the Staphilococcus coagulase (+)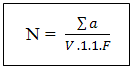
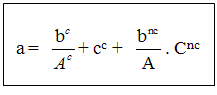 Ac: many mended characteristic coloniesAnc: many mended noncharacteristic coloniesbc: many colonies of positive characteristics of supposed Staphilococcabnc: colonies number noncharacteristic of supposed Staphilococca positiveDC: many colonies characteristic of Staphilococca positive supposed for limpSum of the columns of positive Staphilococca with coagulase identified in two limp.F: bypass ratio with the 1st dilution V: volume spread out over each limps d) Method of enumeration of total aerobic flora mesophile One takes 1 ml of the two marine solutions and his decimal dilutions coming from the two differents cultivation (Mn and MnA). They are put in limp of Petri. After one makes run 15 ml of PCA in limps and the unit is mixed while turning at least 6 times. After the mixture, let solidify the culture. Incubation is done with 30°C during 72 hours [3-5]. e) Method of enumeration of yeasts and mouldsOne takes 0.1 ml of solutions marinates (SM) and his decimal dilutions of the two different cultures (Mn and MnA). The unit is inoculated on the surface of limp of Petri. Container of cultivation OGA. They are incubated at room temperature (25°C) during 5 days. The reading of the colonies is between 15 and 300 [4, 5]. f) Method of enumeration of the anaerobic bacteria sulfitoreducerOne takes 5 ml of the marine solution (SM) of the two cultures (Mn and MnA) and to put in a tube of 20 mm. After, make a dilution of 10-1 and take 5 ml of the dilution of the two cultivation and put in a tube of 20 mm. Run in a tube approximately 20 ml of TSC and the unit is carefully mixed while turning the wrist at least 8 times. After this mixture, let solidify them. Incubation is done with 37°C during 24 to 48 hours in the study. g) Method of enumeration of Escherichia coli-glucuronidaseOne takes 10 ml of SM of the two different cultures and to put in six limp of Petri in the proportion of 2 limp X 1.6 ml and 4 limp X 1.7 ml.Secondly, one takes 10 ml of decimal dilution of the two different cultivation (Mn and Mn A) and to put in six limp of Petri in the proposal of 2 limps X 1.6 ml and 4 limp X 1.7 ml. In two cultures, make run approximately 15 ml of cultivation TBX in limp of Petri. The unit is mixed while turning at least 6 times the wrist. Let it solidify, the incubation is made with 44°C during 24 hours in the drying oven [5].h) Method of enumeration of coliforms totalOne takes 2 X 1 ml of SM coming from the two different cultivation (Mn and Mn A). They are senescences in two limps of Petri.Then, make a dilution 10-1 and take 2 X 1 ml of two dilutions in different cultures to sow in 2 limp of Petri.In two cultures, make run approximately 15 ml of culture VRBL. The unit is mixed while turning at least 6 times the wrist. Let the unit solidify itself and after addition a second layer of in-top culture. Let it solidify, the incubation is done with 30°C during 24 hours in the drying oven [3]. i) Method of enumeration of positive Staphilococca coagulasesOne takes 0.1 ml of SM coming from the two different cultivation (Mn and MnA). And make run in limps of Petri containing of cultivation BP (Baird-Parker agar). Incubation is done with 37°C during 48 hours [2-5].j) Method of enumeration of Salmonella
Ac: many mended characteristic coloniesAnc: many mended noncharacteristic coloniesbc: many colonies of positive characteristics of supposed Staphilococcabnc: colonies number noncharacteristic of supposed Staphilococca positiveDC: many colonies characteristic of Staphilococca positive supposed for limpSum of the columns of positive Staphilococca with coagulase identified in two limp.F: bypass ratio with the 1st dilution V: volume spread out over each limps d) Method of enumeration of total aerobic flora mesophile One takes 1 ml of the two marine solutions and his decimal dilutions coming from the two differents cultivation (Mn and MnA). They are put in limp of Petri. After one makes run 15 ml of PCA in limps and the unit is mixed while turning at least 6 times. After the mixture, let solidify the culture. Incubation is done with 30°C during 72 hours [3-5]. e) Method of enumeration of yeasts and mouldsOne takes 0.1 ml of solutions marinates (SM) and his decimal dilutions of the two different cultures (Mn and MnA). The unit is inoculated on the surface of limp of Petri. Container of cultivation OGA. They are incubated at room temperature (25°C) during 5 days. The reading of the colonies is between 15 and 300 [4, 5]. f) Method of enumeration of the anaerobic bacteria sulfitoreducerOne takes 5 ml of the marine solution (SM) of the two cultures (Mn and MnA) and to put in a tube of 20 mm. After, make a dilution of 10-1 and take 5 ml of the dilution of the two cultivation and put in a tube of 20 mm. Run in a tube approximately 20 ml of TSC and the unit is carefully mixed while turning the wrist at least 8 times. After this mixture, let solidify them. Incubation is done with 37°C during 24 to 48 hours in the study. g) Method of enumeration of Escherichia coli-glucuronidaseOne takes 10 ml of SM of the two different cultures and to put in six limp of Petri in the proportion of 2 limp X 1.6 ml and 4 limp X 1.7 ml.Secondly, one takes 10 ml of decimal dilution of the two different cultivation (Mn and Mn A) and to put in six limp of Petri in the proposal of 2 limps X 1.6 ml and 4 limp X 1.7 ml. In two cultures, make run approximately 15 ml of cultivation TBX in limp of Petri. The unit is mixed while turning at least 6 times the wrist. Let it solidify, the incubation is made with 44°C during 24 hours in the drying oven [5].h) Method of enumeration of coliforms totalOne takes 2 X 1 ml of SM coming from the two different cultivation (Mn and Mn A). They are senescences in two limps of Petri.Then, make a dilution 10-1 and take 2 X 1 ml of two dilutions in different cultures to sow in 2 limp of Petri.In two cultures, make run approximately 15 ml of culture VRBL. The unit is mixed while turning at least 6 times the wrist. Let the unit solidify itself and after addition a second layer of in-top culture. Let it solidify, the incubation is done with 30°C during 24 hours in the drying oven [3]. i) Method of enumeration of positive Staphilococca coagulasesOne takes 0.1 ml of SM coming from the two different cultivation (Mn and MnA). And make run in limps of Petri containing of cultivation BP (Baird-Parker agar). Incubation is done with 37°C during 48 hours [2-5].j) Method of enumeration of Salmonella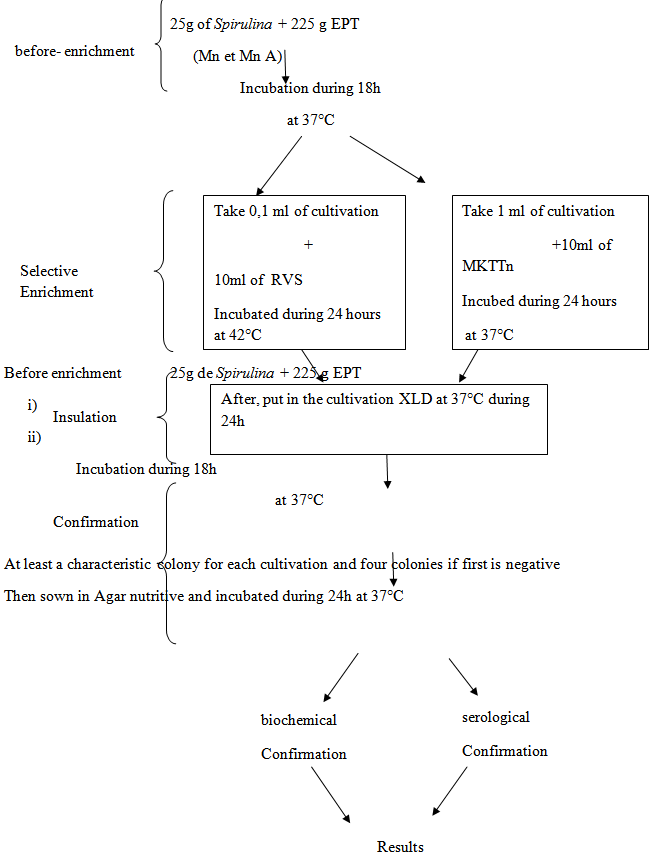
5. Results of Analyses
- Qualitative analysis before the conservation
|
|
|
|
|
|
|
|
|
|
|
|
6. Discussion
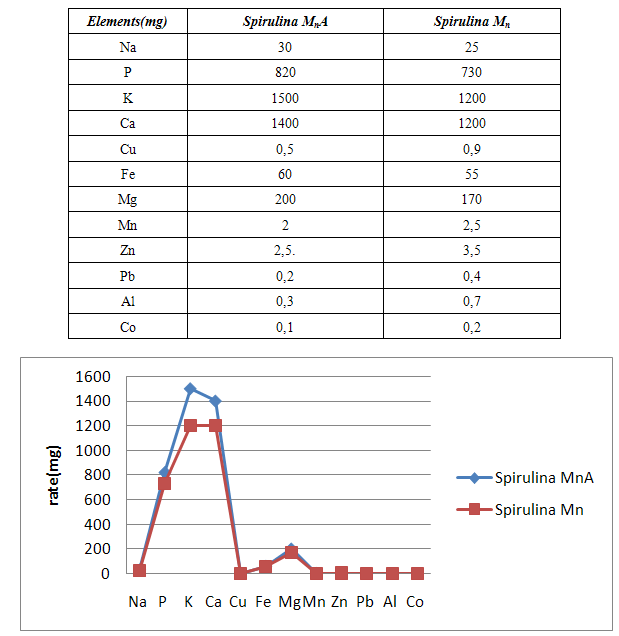
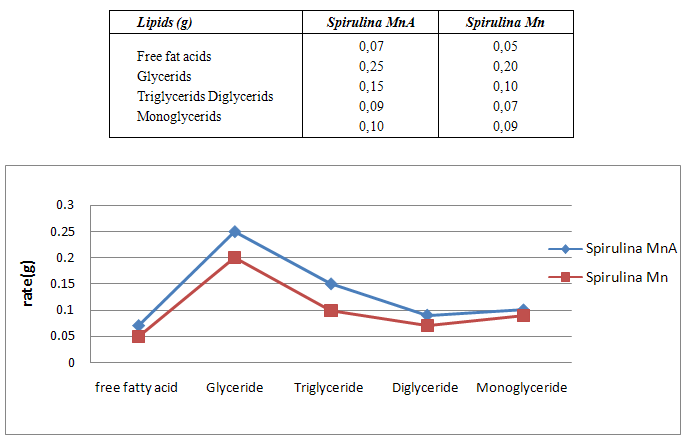
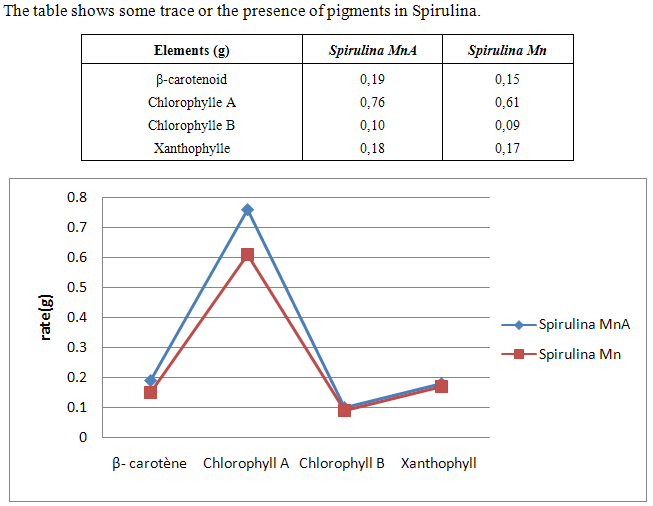
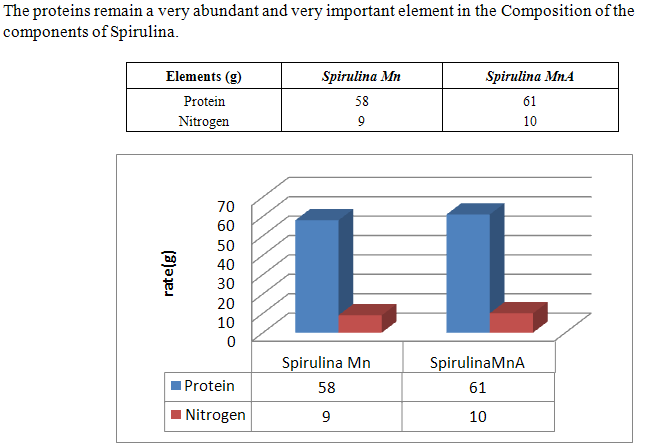
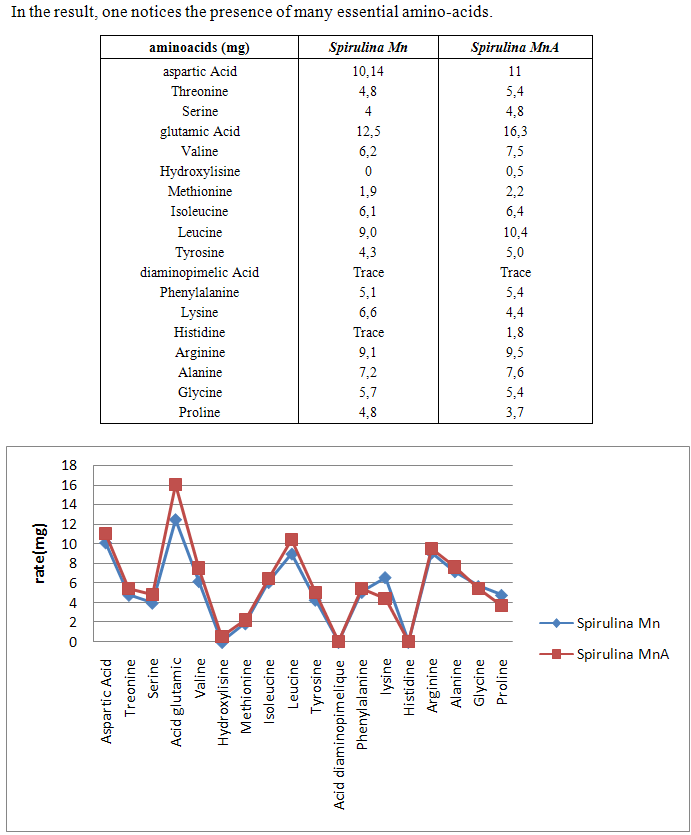
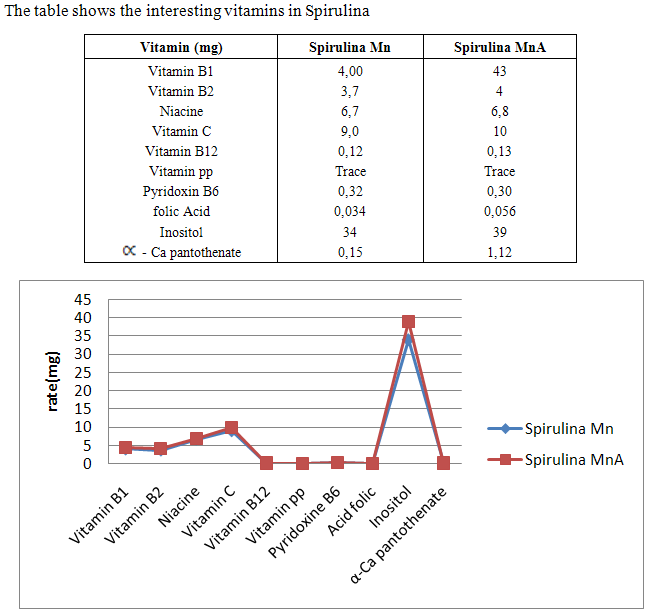
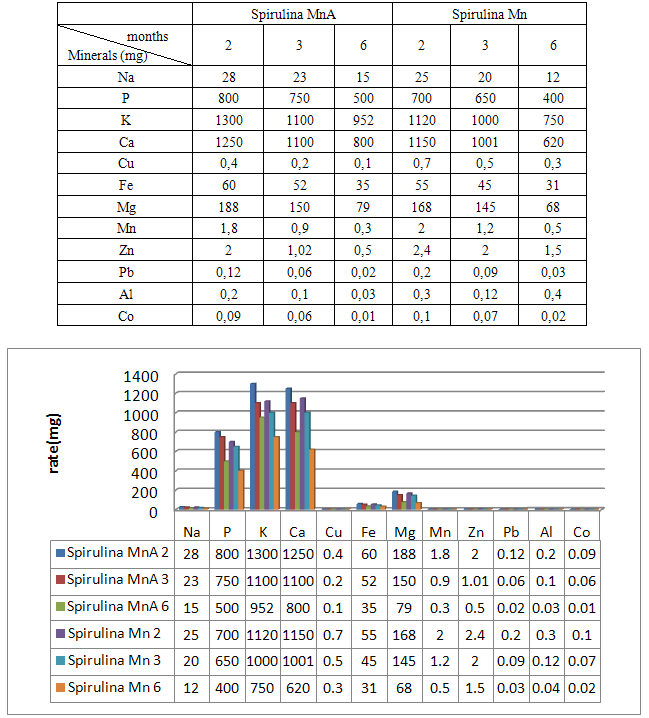
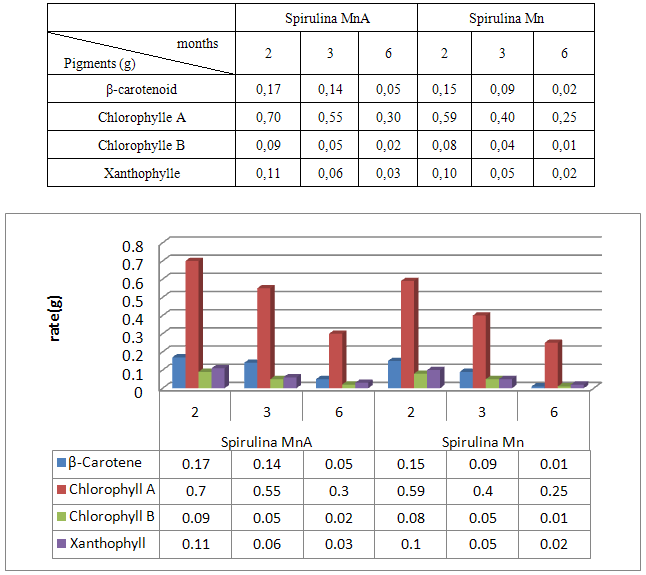
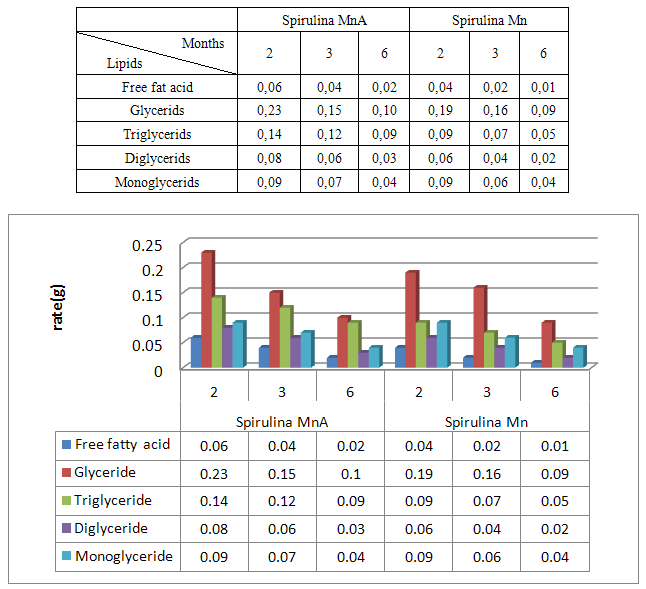
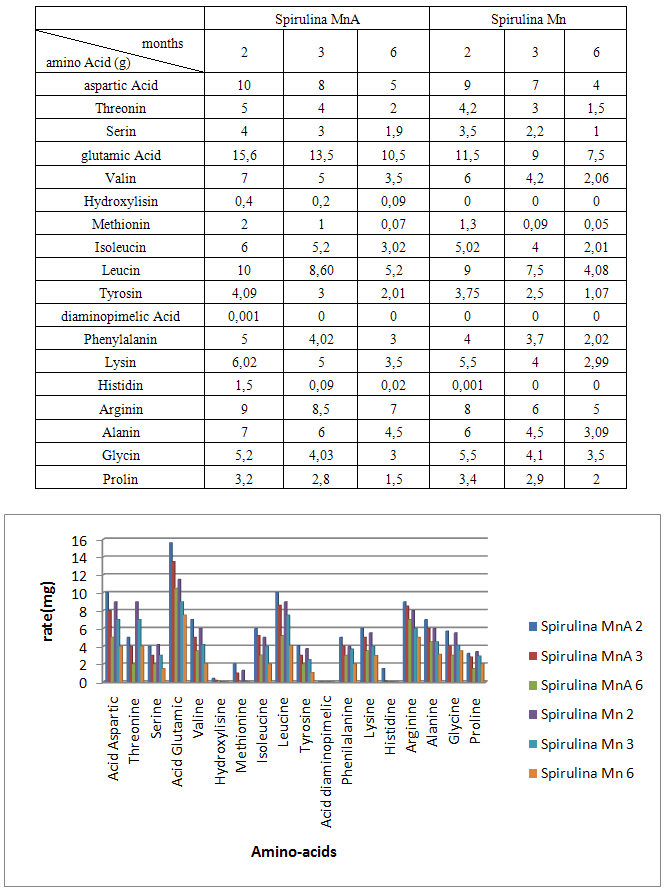
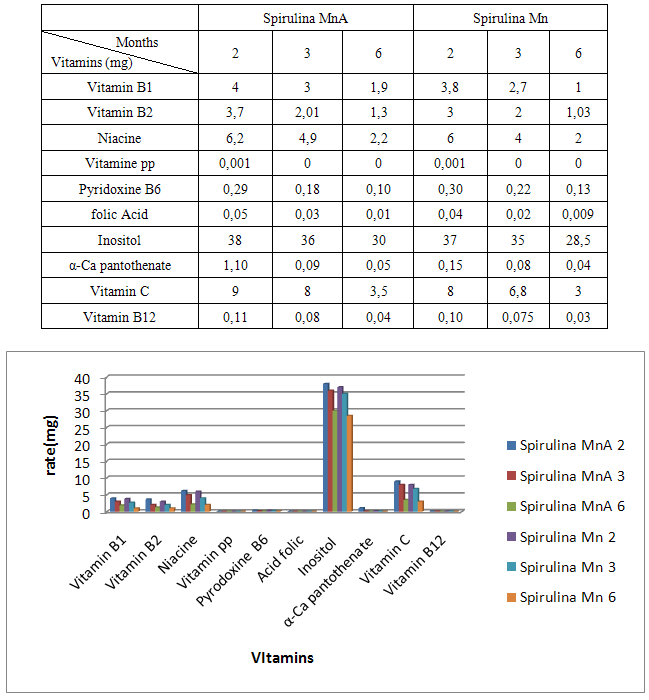
- Spirulina is a very complete food, but one some remark on the level of the two different cultivation. The rates of heavy metals are increased in the cultivation of Spirulina Mn compared to the Spirulina MnA. Therefore, the difference comes from sea water and is in hiding. Even of another element like the pigments, the amino-acids, the lipids and the protein are important in the natural cultivation compared to the improved natural cultivation. The microbiological analysis show that the number of bacteria in the improved natural environment is satisfactory compared to the standard of European Union. On the level of conservation, Spirulina loses almost 3/4 of value of the components after 6 months of conservation. The fall of this rate comes from mode of conservation and preparation. Therefore, for the cultivation, the natural environment is advised for the interested party with the cultivation of Spirulina. Our study is made in the North of Madagascar, therefore the results are very satisfactory that it is on the level of the physicochemical parameters, that it is on the level biochemical parameters.
7. Conclusions
- The area of Sambirano is very favorable to the cultivation of Spirulina. The climates, salinity, the pH, the structure of the ground allow the cultivation of Spirulina. The results show that the rates of the components are not differing of those from the South of Madagascar. Therefore, to ensure and stabilize the components, it is necessary to set up the method HACCP and the strict method of conservation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML