-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2015; 5(1): 53-58
doi:10.5923/j.food.20150501.07
Effects of IP6 and Sweet Potato (Ipomoea batatas) Phytate on Serum, Liver and Faecal Lipids in Rats
Lowell L. Dilworth1, Felix O. Omoruyi2, Helen N. Asemota3
1Department of Pathology, The University of the West Indies Mona Campus, Kingston, Jamaica
2Department of Basic Medical Sciences, The University of the West Indies Mona Campus, Kingston, Jamaica
3The Biotechnology Centre, The University of the West Indies Mona Campus, Kingston, Jamaica
Correspondence to: Lowell L. Dilworth, Department of Pathology, The University of the West Indies Mona Campus, Kingston, Jamaica.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Dyslipidemia is associated with coronary heart disease. It is known that phytate supplementation reduceshepatic triglyceride and cholesterol concentrations in rats. Here, we investigated the effects of sweet potato phytate extract or IP6 on liver, serum and faecal lipid levels as well as intestinal lipase activity in rats fed zinc deficient of zinc sufficient diets. There were significant increases in intestinal lipase activity in the test group fed diets supplemented with sweet potato phytate extract only, compared to other groups. Conversely, faecal triglyceride concentrations were significantly reduced in this group compared to other test groups. Faecal cholesterol output was significantly increased in rats fed diets supplemented with phytate extract only compared to other groups. Total serum lipids did not vary among the groups, however, significantincreases in both serum triglyceride and HDL concentrations were observed in test animals compared to control groups. Liver phospholipid concentrations were found to be significantly higher in animals that consumed zinc supplemented diets compared to those on zinc deficient diets. Phytates may induce hypolipidemia by upregulating intestinal lipase activity resulting in increased faecal cholesterol output and reduced serum lipid levels. Increased serum HDL concentration in rats fed phytate suggests that this supplement may possess cardioprotective properties.
Keywords: Lipids, IP6, Phytic acid, Rats, Sweet potato
Cite this paper: Lowell L. Dilworth, Felix O. Omoruyi, Helen N. Asemota, Effects of IP6 and Sweet Potato (Ipomoea batatas) Phytate on Serum, Liver and Faecal Lipids in Rats, International Journal of Food Science and Nutrition Engineering, Vol. 5 No. 1, 2015, pp. 53-58. doi: 10.5923/j.food.20150501.07.
Article Outline
1. Introduction
- Disruptions in normal metabolic processes oftentimes result in metabolic consequences in which dyslipidemia may be a commonly observed event. Dyslipidemia may also exist on its own as a result of genetic abnormalities identified and grouped under the Fredrickson’s classification, or may develop as a complication of an already existing pathology for example diabetes mellitus. The recognized association of dyslipidemia with coronary heart disease has prompted research in the area with much attention focusing on pathogenesis and treatment. While statins remain the drugs of choice in some countries, other research geared towards developing alternative treatment regimens is ongoing. IP6, sometimes referred to as phytate, is a naturally occurring compound which has shown promise in treating dyslipidemia. Research shows that phytate supplementation reduces hepatic triglyceride and cholesterol concentrations in rats [1]. Other research shows that phytate supplementation lowers serum and liver lipid levels but the effects on triacylglycerol levels were minimal [2]. While phytates are predominantly known for their antioxidant, anticancer and hypoglycemic properties, other studies are needed to ascertain the mechanisms by which they are able to control dyslipidemia [3] [4]. Phytates are known chelators of divalent minerals, but it is theorized that they may also bind to specific enzymes or enzyme bi-products thereby affecting the rate of removal of these products from the gastrointestinal tract. This might be a potential mechanism by which serum lipids can be modulated by phytates and this warrantsfurther investigations.Tuber crops including yams, potato and cassava are widely consumed throughout the tropics. It is however thought that sweet potato while highly nutritious, is underutilized as seen by its reduced consumptioncompared to other tuber crops. This project therefore sought to highlight some properties of a bioactive compound found in sweet potato with a long term view of increasingcultivation and consumption of the crop. Once accepted into mainstream, it can serve as a cheap highly nutritious source of food for millions in the developing world. In addition, it may also be exploited for health benefits since this crop is a reservoir for a myriad of bioactive compounds that may possess beneficial nutraceutical properties. Keeping all this into consideration, the aim of the present study was to assess lipid metabolism in rats fed diets containing 5% IP6 or crude sweet potato phytate extract containing 5% phytate in order to assess the effects of these compounds in the pure and crude form on lipid metabolism.
2. MaterialsandMethods
- Fresh matured sweet potato (Ipomoea batatas) tubers were harvested from a local farm in the Parish of Manchester, Jamaica. Tuber samples were washed with distilled water, oven dried at 65℃ to constant weight and ground into fine powder.
2.1. Extraction and Determination of Phytic Acid
- Phytic acid was extracted by a modification of an established method [5]. A known amount of sweet potato was blended for 5 min with 10% tri-chloro acetic acid in a Waring Blender. The slurry was filtered by suction in a sintered glass funnel and the residue washed successively with known volumes of 5% tri-chloro acetic acid. Filtrates were combined, neutralized with 5M NaOH and freeze-dried for use as phytic acid extract. Phytic acid content of the extract was determined by the method of Holt [6] as described by Davies and Olpin [7]. Phytic acid was purchased from Sigma-Aldrich, St. Louis, MO, USA.
2.2. Feeding Experiments
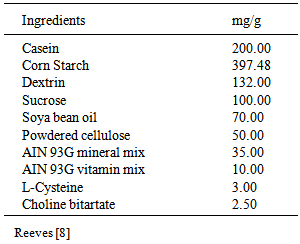
- The experimental animals were 36 adult Wistar rats assigned by weight into 6 groups of 6 rats each; average body weights 236.4g. Diets were prepared according to standard methods of diet preparations, AIN-93G Purified Rodent diet. AIN-93G Vitamin mix and AIN-93G Mineral mix [8]. Test groups were fed as follows: Formulated Diet (D), formulated diet supplemented with zinc (D+Zn), formulated dietsupplemented with zinc plussweet potato phytate extract (D+Zn+PE) and formulated diet supplemented with zinc pluscommercially available phytate (D+Zn+IP6). D+Zn+PE and D+Zn+IP6 were formulated to simulate the phytate to zinc ratio (18:1) observed in sweet potato commonly consumed in the Caribbean. The two remaining groups were fedformulated dietand sweet potatophytate extract only (D+PE) and formulated dietplus commercially available phytate (D+IP6) respectively. The levels of phytate and zinc in the extract were 9.46 mg/g and 0.02 mg/g respectively. Zinc was added as part of the mineral mix to the diets of groups D+Zn, D+Zn+PE and D+Zn+IP6 animals, in the form of zinc carbonate at a concentration of 1.65 mg/Kg [8].The specific composition of the diets is depicted in table 1. Zinc levels in groups D, D+PE and D+IP6 were negligible as determined by Atomic Absorption Spectrophotometry. The respective diets fed to different groups are listed below.Rats were housed in stainless steel cages in a room kept on a 12 hour light-dark cycle and allowed access to their respective diets and water freely. The cages were cleaned daily. Prior to the start of the experiment, all the animals were fed the control diet (formulated diet with zinc) for one week to allow for acclimatization to the new diet. The rats were then fed their respective test diets for three weeks. Body weight changes and total food intake were recorded weekly. At the end of the experimental period, the rats were sacrificed by decapitation following an overnight fast. Approval for the study was obtained after presentation of the protocol to the Board of the Department of Basic Medical Sciences, University of the West Indies, Mona Campus, Jamaica.
|
2.3. Intestinal Lipase Assay
- Intestinal lipase activity was determined by amodification of the method of Percival and Schneeman [9]. Briefly, the reaction medium containing 1.0 mL of triolein substrate, 1mL of sodium taurocholate, 0.2 mL ammonia buffer, and 2.0 mL of 0.1M CaCl2 was warmed gently with shaking until homogenous. 0.2 mL of 1.0% phenolphthalein was added followed by 0.8 mL of water and 0.8 mL intestinal homogenate then the medium was mixed and incubated for an hour. At the end of incubation, 5 mL sample was withdrawn and 25 mL of ethanol-ether (9:1 v/v) mixture was added to stop the reaction. The solution was immediately titrated with 0.02N standard KOH. Titrations were done in triplicate. Protein content of homogenate was determined by the method of Bradford [10]. The specific activity of the enzyme was expressed as µmoles of fatty acid liberated per hour per mg protein.
2.4. Determination of Total Lipids
- Total liver lipids were extracted using a modification of the method of Blight and Dyer [11], as described below. One gram of liver was added to10 mL water and methanol-chloroform (2:1 v/v) mixture and blended for 2 minutes at room temperature. The homogenate was centrifuged and the supernatant decanted. The residue was re-extracted with a known volume of methanol-chloroform-water (2:1:0.8 v/v/v). After centrifugation, the combined supernatants were diluted with equal volumes each of chloroform and water and the phases separated with a separatory funnel. The chloroform was allowed to evaporate. Total lipid weight was determined gravimetrically.
2.5. Determination of Total Cholesterol
- Determination of total blood, liver and faecalcholesterol levels was carried out by a modification of methods described by Zlatkis et al. [12]. Plasma or liver homogenate (0.1 mL) was added to 3 mL of glacial acetic acid. Two milliliters of the color reagent (containing ferric chloride and sulfuric acid) was added and the content of each tube mixed thoroughly. A range of standardcholesterol solutions and a blank were similarly treated and absorbance read at 560nm.Plasma cholesterol levels in each blood sample were determined by extrapolation from the standard cholesterol curve.
2.6. Determination of Liver and Plasma HDL
- Plasma HDL was determined by the methods of Lopes-Virella et al. [13]. Phosphotungstic acid and magnesium chloride were used to precipitate out low-density lipoproteins and very low density lipoprotein cholesterol. High-density lipoprotein was then quantified as the amount of cholesterol remaining in the supernatant. In summary, 0.1 mL of 5% phosphotungstic acid reagent was added to 0.1 mL of plasma or liver homogenate and the mixturevortexed. 0.05 mL of a 2M magnesium chloride solution was added and the mixture centrifuged at 1500x g at 4℃ for 30 minutes. The clear supernatant was carefully removed and cholesterol was determined in the supernatant using the method of Zlatkis et al. [12].
2.7. Determination of Triglycerides
- Triglycerides were determined by a modification of method as described by of Gottfried and Rosenberg [14]. Normal heptane was added to serum, liver or faecal homogenate followed by Isopropanol and1.0 mL of concentrated sulfuric acid. Samples were mixed and allowed to separate. A range of standard triolein samples and a blank were at the same time treated similarly. Heptane extract was saponified by adding 2.0 mL of isopropanol and one drop of potassium hydroxide. The mixture was mixed and incubated at 70℃ for 10 minutes. Sodium metaperiodate reagent and acetyl acetone reagent were added and the resulting solution mixed, and incubated for another 10 minutes at 70℃. The mixture was allowed to cool at room temperature and absorbance read at 425 nm against a blank. The amount of triglycerides present in each serum sample was determined by extrapolation from the triolein curve.
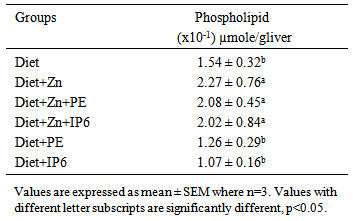
2.8. Phospholipid Assessment
- Liver phospholipids were separated and quantified by standard methods [15, 16, 17]. Briefly, 1 mL of lipid extract was placed in a test tube and evaporated to dryness. Samples were digested on a sand bath with 0.5mL of 70% perchloric acid at about 200-300℃ for 20-30 minutes and the digest made up to 10 mL with distilled water.0.5 mL of 2.5% ammonium molybdate was added followed by 0.5 mL of 0.2% ascorbic acid and absorbance of the solution read at 625nm. Statistical analyses were expressed as means ± standard error of the mean (SEM). Analysis of variance was used to test for significance difference between the groups. Duncan Multiple Range Test was used to test for significance difference among the means and p<0.05 was taken as significant [18].
3. Results
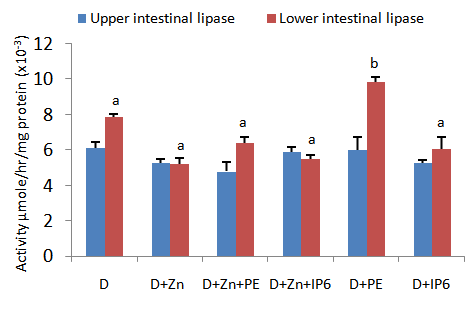
3.1. Intestinal Lipase Activity
- Figure 1 shows intestinal lipase activity for rats fed diets supplemented with sweet potato phytate extract or IP6. Lower intestinal lipase activity was significantly higher in rats fed a formulated diet supplemented with sweet potato phytate extract (D+PE) compared to all other groups. Results show that upper intestinal lipase activity did not vary significantly among the test groups.
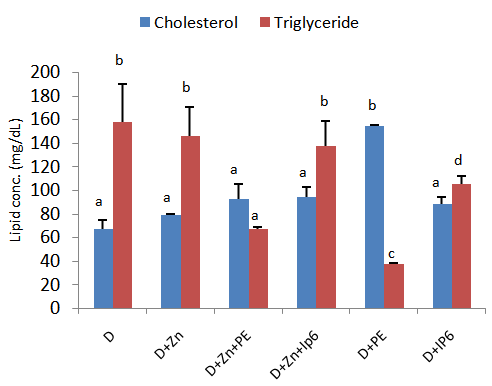
3.2. Faecal Lipid Levels
- Figure 2 depicts faecal cholesterol and triglyceride levels in test animals fed diets supplemented with IP6 or sweet potato phytate extract. Faecal cholesterol output was found to be significantly increased in rats fed diets supplemented with phytate extract only. It was observed that faecal triglyceride concentrations in this same group were significantly reduced compared to all other test groups. Faecal triglyceride concentrations in the other test group fed diets supplemented with sweet potato phytate extract (D+Zn+PE) was significantly higher than those observed in group D+PE. Faecal triglycerides in these two groups were however significantly lower that the concentrations observed in all other test groups.
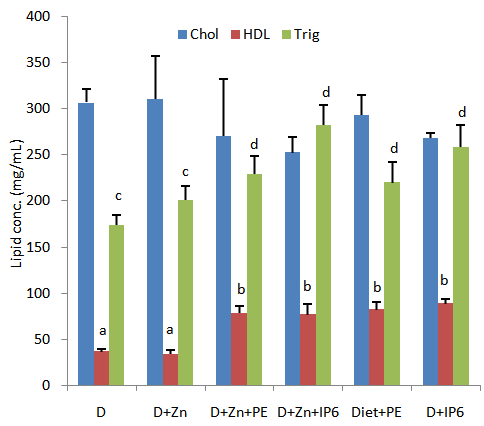
3.3. Blood Lipid Profile
- Figure 3 shows blood lipid profile of rats fed diets supplemented with IP6 or sweet potato phytate extract. Blood triglyceride concentrations were observed to be significantly increased in rats fed IP6 or sweet potatophytate supplemented diets compared to the control groups. A similar pattern was observed for blood high density lipoprotein concentrations wherein animals which consumed diets without phytate or IP6 supplementation (D and D+Zn respectively) displayed significantly reduced blood HDL compared to test animals. Total cholesterol concentrations did not vary significantly among test groups.
3.4. Hepatic Lipids
- Figure 4 shows results from studies of hepatic total and high density lipoprotein cholesterol in rats fed diets supplemented with IP6 or sweet potato phytate extract. Fluctuations in serum total cholesterol were observed among all groups assessed by these variations were not significant at the p<0.05 level. Similarly blood high density lipoprotein cholesterol concentrations did not vary significantly among the groups assessed.
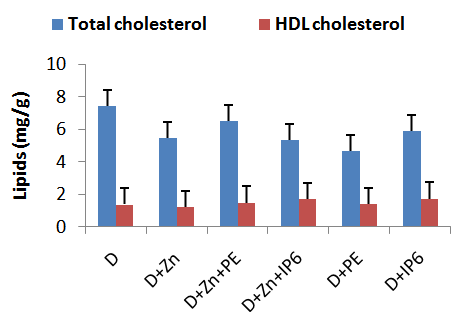 | Figure 4. Total and HDL cholesterol concentration in the liver of rats fed sweet potato phytate extract or IP6. Values are expressed as mean ± SEM where n=3 |
|
4. Discussion
4.1. Effects of IP6 and Phytate Extract on Lipid Metabolism
- There were observed significant increases in intestinal lipase activity in rats fed diets supplemented with crude sweet potato phytate extract (D+PE) compare to all other groups. This resulted in increased fat digestion that eventually resulted in significantly increased total faecal cholesterol output in those animals. Intestinal lipase has high specificity for triglycerides, hence any increase in its activity should result in reduced triglyceride output arising from increased metabolism of the substrate [19]. This observation was reflected in faecal triglyceride levels in animals with increased lipase activity as faecal samples from these rats contained significantly reduced triglycerides compared to all other groups. By virtue of its actions, it was expected that increased intestinal lipase activity would have significantly reduced blood triglycerides levels in the test animals. While serum triglyceride concentrations in rats fed diets supplemented with crude sweet potato phytate extract were lower than levels observed in other test groups, the difference was not significant and more importantly, blood triglyceride concentrations observed in test groups were significantly higher than values reported for control groups. This suggests that in a short term study, low dose phytates in the form of IP6 or sweet potato phytate supplementation may not independently reduce serum triglyceride concentrations in rats. This observation was also made by Lee et al. [2], who suggested that low doses of phytates are not effective in significantly reducing serum triglycerides. It is not known whether higher doses of IP6 or phytate extract are capable of doing this. Further studies are needed to elucidate this.It was expected that an increase in intestinal lipase activity resulting in increased rates of triglyceride metabolism would lower serum triglycerides and ultimately contribute to reductions in total blood cholesterol levels. This observation was not made as the blood cholesterol output in animals fed sweet potato phytate extract which had the highest lipase activity, was not significantly lower than the control or other test groups. This may again be attributed to the ineffectiveness of phytates on intestinal lipase at the dosage used. The observed reduction in total blood cholesterol in test groups fed IP6 or sweet potato phytate extract compared to controls was reflected in slightly increased faecal output in these groups. These results indicate that while phytate supplementation may increase serum triglycerides, they may also play a role in reducing total serumcholesterol. The observation that different forms of phytates possess hypocholesterolemic actions was also made by other researchers [2, 20]. It is theorized that in the long term, phytates may bind to the charged regions of these bile salts followed by their removal from the gut. This may directly lead to reduced blood cholesterol as primary bile salts are synthesized directly from cholesterol [21]. A similarproposal was put forward by Yuangklang et al. [22], who found that sodium phytate supplementation enhanced faecal bile acid excretion. This observation was not made in this short term study.Reverse cholesterol transport by HDL is thought to be primary mechanism by which HDL is protective against artherosclerosis and other related cardiovascular events [23]. Most drugs used in cardiovascular disease management are however shown to reduce both LDL and HDL. Results from this study suggest that sweet potato phytate extract as well as commercially available IP6 may have protective roles against coronary heart disease, as consumption of phytate in either form by rats lead to significantly increased plasma HDL levels. The mechanism is unclear but it may be related to reduced HMG-CoA reductase activity [24]. Further studies aimed at assessing the effects of crude phytate extract or IP6 on this enzyme are needed. This is the first known study highlighting some cardioprotective benefits of IP6 and crude sweet potato phytate consumption with respect to maintenance of high levels of blood HDL concentrations. In this study, animals with elevated blood HDL concentrations also displayed increased liver HDL thought not to significant levels. High serum HDL is desirous as a result of its positive association with lowered risk of artherosclerosis [25]. Maintaining high serum HDL concentrations therefore remains one of the goals in treating dyslipidemia. The role of dietary phytates in achieving this feat needs to beassessed further especially in light of the new thrust towards use of fresh tubers and their crude extracts as nutraceuticals.In this study, supplementation of the diet did not significantly alter total liver cholesterol concentrations. Supplementation of the diets with zinc also had no significant effect on liver cholesterol levels. This study is in line with previous studies which demonstrated that lowlevels of phytates may not significantly influence hepatic activity of carnitine palmitoyltransferase which is the rate-limiting enzyme of fatty acid oxidation [26]. Other studies however show that at a dose of around 0.035%, dietary phytates may accelerate hepatic lipogenesis thereby reducing the risk of fatty liver development [27].
4.2. Effects of Phytates on Phospholipids
- Zinc is essential in maintenance of cellular integrity. It is important in the maintenance of the integrity of biological membranes resulting in their protection against oxidative injury and is also an important cofactor for many hepatic enzymes [28, 29]. Research by Khoja et al. [28], shows that severely zinc deficient rats had low liver phospholipids. Our research corroborates this observation as there were significant increases in liver phospholipids observed in all groups administered zinc-containing diets, compared to the other groups. This indicates that sufficient dietary zinc is required for maintaining optimal phospholipid concentrations that contributes positively to preservation of cell membrane integrity.
5. Conclusions
- Low dose phytate supplementation in the form of a crude sweet potato extract or the purified compound IP6, may not independently lower serum total cholesterol or triglyceride concentrations. Consumption of these products by rats however significantly increased serum HDL concentrations indicating that both the purified IP6 as well as the crude sweet potato extract may possess cardioprotective properties and should be explored further. Increased Intestinal lipase activity resulted in increased triglyceride metabolism with subsequent reductions in faecal triglyceride and increased total faecal cholesterol. The concentrations of liver lipids in this study were unaffected by inclusion of IP6 or unpurified sweet potato phytate in the diet. Adequate zinc supplementation of the diet is suggested for maintenance of cellular integrity especially.
ACKNOWLEDGEMENTS
- The authors wish to thank the postgraduate research and publications grant committee at the University of the West Indies Mona campus for funding this research. Funding source had no other contribution to the paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML