-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2015; 5(1): 1-7
doi:10.5923/j.food.20150501.01
Physical and Chemical Characterization of Roasted Cashew Nut (Anacardium occidentale) Flour and Oil
Ogungbenle H. N. , Afolayan M. F.
Department of Chemistry, Ekiti State University, Ado-Ekiti, Nigeria
Correspondence to: Ogungbenle H. N. , Department of Chemistry, Ekiti State University, Ado-Ekiti, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
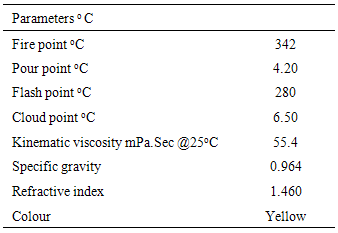
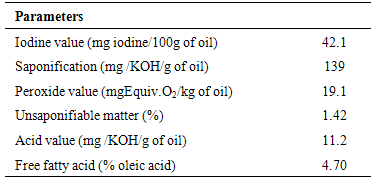
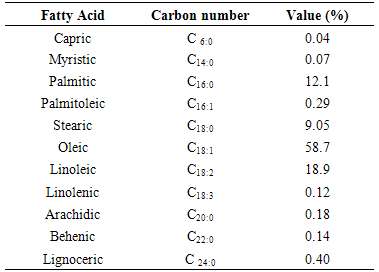
Proximate and physico chemical properties of roasted cashew nut (Anarcadium occidentale) flour and oil were investigated. The result showed that moisture (5.90 %), crude fat (42.9 %), crude protein (26.1 %), crude fibre (3.11 %) and carbohydrate (19.0 %). The physico chemical properties of roasted cashew nut oil were as follow; colour (yellow), refractive index (1.460), specific gravity (0.964), acid value (11.2 mgKOH/g), saponification value (139 mgKOH/g), iodine value (42.1 mg iodine/100g) and free fatty acid (4.70 %). This is an indication that the oil is non-drying, edible and may not be useful for soap making. The fatty acid values were: myristic acid (0.07 %), palmitic acid (12.06 %), palmitoleic acid (0.29 %), stearic acid (9.05 %), oleic acid (58.7 %), linoleic acid (18.9%), linolenic acid (0.12 %), arachidic acid (0.18 %), behenic acid (0.14 %) and lignoceric acid (0.40 %). Oleic acid was the highly concentrated while linoleic acid was the least concentrated. The percentage oil yield makes the nut a good source of oil. The total saturated fatty acid was 21.6 % while the total unsaturated fatty acid was 78.1%. It is an indication that the oil is economically viable.
Keywords: Proximate, Physico chemical, Fatty acid, Roasted, Cashew, Oil
Cite this paper: Ogungbenle H. N. , Afolayan M. F. , Physical and Chemical Characterization of Roasted Cashew Nut (Anacardium occidentale) Flour and Oil, International Journal of Food Science and Nutrition Engineering, Vol. 5 No. 1, 2015, pp. 1-7. doi: 10.5923/j.food.20150501.01.
Article Outline
1. Introduction
- Global trends in acute food shortages in both developed and developing countries demand that food scientists should intensify efforts to salvage the situation by providing nutritional information to educate the teeming population so as to expose some under utilized legumes. Plant sources of protein are the major avenue for protein intake in some developing countries [1]. Increase in the world population has contributed to substantial decline in per capital supply of conventional protein foods [2]. This has led to over dependency of people on starchy foods in developed and under developed countries [3]. Cashew (Anacardium occidentale) is a tree in the family Anacardiaceous. It is originally native to North-east of Brazil and spreads across Africa and West indies [4, 5]. It is a drought resistant tree widely grown in tropical climates between the tropics of Cancer and Capricorn basically for its cashew apples and nuts. The cashew seed is heart like shaped and the tree grows well in a variety of soils and climatic conditions where other commercial trees would not grow. It is edible and has a ‘’sweet’’ taste. The pulp of the cashew apple is very juicy, fragile and unsuitable for transport but can be used as fruit drinks with refreshing taste [5, 6, 7].The motivation to do this work is that cashew is common and widely grown in Africa but yet there is limited information on the nutritional composition, utilization and physico chemical properties; therefore, compositional data from the study would provide scientific knowledge on the nutritional status of roasted cashew nut flour and oil and this would further expose its potentials as food. The data obtained would also add or contradict the existing ones if available. Ogungbenle [3] reported the proximate, minerals, anti nutrients and amino acid of the kernel. This paper reveals the proximate, physico chemical, kinematic viscosity and fatty acid of the oil.
2. Materials
- Cashew nut seeds were purchased from Ado-Ekiti Central market, Ekiti State, Nigeria in Africa continent. The seeds were roasted in a Cabolite oven at regulated temperature of 150-200℃ and then removed from the pods. The roasted seeds were screened to remove the bad seeds. The remaining good seeds were dehulled and blended into fine flour. The oil from the cashew nut flour was extracted using petroleum ether of Analar grade (British Drug House, London) boiling range 40-60℃.
3. Methods
3.1. Proximate Analysis
- The moisture and ash contents determined using the air oven and dry ashing method Pearson [8]. The sample was analyzed for crude fat, fibre and crude protein according to the methods described by AOAC [9]. Nitrogen was determined by micro-Kjedahl method [9] and the percentage nitrogen was converted to crude protein by multiplying by a factor of 6.25. The total carbohydrate content was calculated by method of difference as described [3].
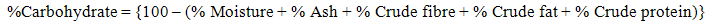 | (1) |
3.2. Physico Chemical Properties
3.2.1. Determination of Saponification Value
- A 2.0ml of the oil sample was added to the 20ml of ethanolic potassium hydroxide in 500ml round bottom flask. The flask with its content was refluxed for 30 minutes. 2ml of phenolphthalein indicator was added and the hot solution was allowed to cool and later titrated against the 0.5M hydrochloric acid. A blank titration was carried out using the same procedure [8, 10].
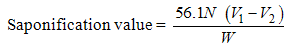 | (2) |
 N = molarity of hydrochloric acid.
N = molarity of hydrochloric acid. V1 = volume of HCl used in the test.
V1 = volume of HCl used in the test. V2 = volume of HCl used in the blank.
V2 = volume of HCl used in the blank. W = weight of sample oil.
W = weight of sample oil.3.2.2. Determination of Peroxide Value
- A 2.0g of the oil sample was weighed into the 200ml conical flask containing 20ml of petroleum ether and heated for 30 seconds in a water bath. 20ml of 50% aqueous solution of potassium iodide and 25ml of distilled water were added. The resulting mixture was titrated with 0.002M sodium thiosulphate solution. During the titration a milky white precipitate was observed and the total disappearance of the precipitate indicated the end point of the titration. The peroxide value of the sample oil was estimated on the basis of the equation below. The same procedure was repeated for the blank [11].
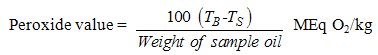 | (3) |
 N = molarity of thiosulphate
N = molarity of thiosulphate TS = volume of thosulphate used in the sample test.
TS = volume of thosulphate used in the sample test. TB = volume of thiosulphate used in the blank.
TB = volume of thiosulphate used in the blank.3.2.3. Determination of Acid Value
- A 5g of the sample oil was weighed into a 250 ml conical flask. 50 ml of hot neutralized alcohol was measured into the flask. The content in the flask was boiled on a water bath, after which 5 drops of phenolphthalein indicator was added into the content of the flask. The mixture was then titrated with 0.1M sodium hydroxide using a burette until a pink colour was observed, indicating the end point [11].
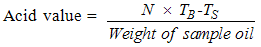 | (4) |
 TS = Titre value of the sample.
TS = Titre value of the sample. TB = Titre value of the blank
TB = Titre value of the blank3.2.4. Determination of Iodine Value
- 0.2g of the sample oil was transferred into a flask containing 10ml carbon tetrachloride. 25ml of Wijs solution was added into the flask containing the sample (Wijs solution consists of iodine monochloride in glacial acetic acid). Blank was prepared. The mixture was stored in a dark place for 30 minutes at temperature of 25oC after which 15ml potassium iodine solution was added along with 100ml of distilled water. The resulting mixture was titrated with 0.1M sodium thiosulphate solution using 2ml of 1% starch indicator. The titration was continued until the blue colour just disappeared, indicating the end point [8, 11].The iodine value was calculated on the basis of the following equation:
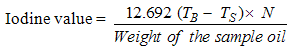 | (5) |
 TS = Titre value of the sample.
TS = Titre value of the sample. TB = Titre value of the blank
TB = Titre value of the blank3.2.5. Determination of Unsaponifiable Matter
- After saponification, 300ml of the mixed solvent of ethanol (70%), toluene (25%) and 5ml oil was added to the packed glass column. It was allowed to run through the column at the rate of 12ml / minute. The glass column was washed with 150ml of the solvent mixture at the same rate. It was concentrated to 25ml using rotary evaporator and then transferred to the tarred dish for evaporation in oven at 105oC for 15 minutes. The dried sample was weighed and titrated for the remaining acids; the weight was corrected for the unsaponifiable matter [9].
3.2.6. Determination of Specific Gravity
- The sample (40ml) was homogenized and poured into a 500ml measuring cylinder gently to avoid air bubbles. The temperature was controlled to avoid drifting in the temperature value. Hydrometer was dipped into the oil carefully to avoid resting on the wall of the cylinder and the reading was then taken [11].
3.2.7. Determination of Refractive Index
- The oil was dried to make it free of moisture. Two drops of the oil was put on the lower prism of the equipment and the prism was closed up. The water was passed through the jacket at 45℃, the jacket was adjusted until the equipment read temperature of 40℃. The light was adjusted and the compensator was moved until a dark border line was observed on the cross wire. The reading on the equipment was recorded [11].
3.2.8. Determination of Kinematic Viscosity
- This was determined by following the method described by Nzikou et. al. [12]. The capillary viscometer tube was used for the determination. The sample was filtered to remove impurities and then introduced into the viscometer and was allowed to stay in a regulated water bath long enough to reach the desired temperature. The head level of the test sample was adjusted to a position in the capillary arm of the equipment to about 5mm ahead of the first timing work. As the sample was flowing freely, the time required for the meniscus to pass from the first time mark to the second was read. The equation used was:
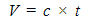 | (6) |
3.2.9. Determination of Flash and Fire Points
- The dried sample was poured into the cup of the tester to the mark and then placed the cup and cover with the left hand pointing toward the left front corner of the test compartment. Stirrer driver was fixed into the tester properly and the resistance thermometer probe connected. Flame and the pilot light were carried out by lighting and the drought screen was closed. The tester was switched on and the heater temperature was regulated to provide homogeneity. The flash occurred when a large flame was observed on the cup and the temperature at which this occurred was recorded as the flash point for the oil sample. The fire point was temperature observed when the oil combustion was sustained after the flash point of the oil sample was recorded [13].
3.2.10. Determination of Pour Point
- The sample was homogenized and poured into the test jar to mark level. The jar was closed tightly with the cork carrying the high pour thermometer that was placed 3mm below the surface of the oil. The disc was placed in the bottom of the jacket and the ring gasket was placed around the jar at the 25mm from the bottom. The test jar was then placed in the jacket. The oil was allowed to cool without disturbance to avoid error. The test jar from the jacket was removed carefully and tilted to ascertain whether there is a movement of the oil. The procedure continued in this manner until a point was reached at which the oil in the test jar showed no movement when the test jar held in a horizontal position for 5 minutes [13].
3.2.11. Determination of Cloud Point
- The determination of cloud point was done using a high precision cloud meter (waveguide sensor total - reflection type), the wave guide sensor have an incidence channel, emergence channel and a detector surface that intersect along the detection surface. The incidence optical fibre connected to the exit of the emergence channel, and a cooling / heating of the waveguide sensor was done within a desired temperature range. The sample was placed on the detection surface and light introduced into the incidence optical fibre. The emergence light from the optical fibre was detected. The wave guide sensor was cooled / heated thereby cooling / heating the sample and the temperature wherein the total reflection of light in the emergence optical fibre as the cloud point of the oil sample [13].
3.3. Fatty Acid Profile
- The fatty acid profile was determined using a method described [14]. The fatty esters analyzed using a PYE Unicam 304 gas chromatography fitted with a flame ionization detector and PYE Unicam computing integrator. Helium was used as carrier gas. The column initial temperature was 150℃ rising at 5℃ min-1 to a final temperature of 200℃ respectively. The peaks were identified by comparison with those of standard fatty acid methyl esters.
4. Results and Discussion
- The results of proximate analysis of the roasted cashew nut flour are shown in Table 1. Most of present proximate results obtained were similar to that reported for cashew kernel [3] but slightly differ from those reported by some previous workers [5, 15, 16, 17]. The moisture content of cashew nut was 5.90 %. This value was higher than that of date palm fruit (5.24%) [18] but lower than those of walnut (11.01%) [19] and velvet tamarind (8.22%) [20]. Ash content of roasted cashew nut presently reported was 2.91%. This value was higher than those of African nut meg (2.27% [21], pearl millet (1.8%) and quinoa (1.2%) [22] but lower than that of benniseed [22], cashew nut may be suitable for animal and human feeds. The values of crude fat and crude protein were: 42.9% and 26.1%. The crude fat was comparable with those values for varieties of underutilized oil legumes that ranged between 43.8-51.9% [23], but higher than those reported for unhulled Bracystegia eurycoma (15.0%), Detarium microcarpum (18.5%) [24] and kidney bean [25]. The value for crude fat presently reported for roasted cashew nut was a little higher than that reported [5, 26]. The differences may be due to analytical conditions involved during analysis, species of the cashew nut, processing and the environment in which they are grown. Crude fat is very important as it helps to increase the mouth feels of foods. This value of fat shows that cashew nut is an oil seed. The high crude protein and crude fat content reported in this work were in agreement with the work of Arogba [27] on cashew and lower than that reported for cashew nut [5]. The crude protein content (26.16%) was higher than those values reported for some flours like Moringa oleifera leaves (3.00%) [28] and Parinari curatellifolia (12.7%) [29]. The crude fibre currently reported for cashew nut (3.11%) was higher than those of kidney bean (2.68%) [25], cowpea (2.10%) [30], cream coat bambara groundnut (2.00%) [15] but the value was lower those of Terminalia catappa oil (4.94%) [12], scarlet runner bean oil [23], velvet tamarind (7.15%) [20] and Cladodes whole flour (CWF) (9.33%) [31]. This observation suggests that the sample would provide good dietary fibre in the diet. It has been observed that polysaccharides also influence digestion and absorption processes in the small intestine. Main effects are exerted in the large intestine [32, 33, 34]. Crude fibre helps in the maintenance of normal peristaltic movement of the intestinal tract hence diets containing lower fibre could cause constipation and eventually lead to colon disease, piles, cancer and appendicitis [35].
|
|
|
|
|
5. Conclusions
- It can be concluded that the roasted cashew nut is a good source of protein and oil. The results obtained from the analyses were compared favourably with conventional edible oils. The high oil yield makes it economically and industrially useful. The oil also exhibits good physical and chemical properties that enable it ranks good among edible oils.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML