-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2014; 4(4): 106-111
doi:10.5923/j.food.20140404.03
Effect of Supercritical Carbon Dioxide on the Enzymes Inactivation in Single-Strength Carrot Juice
Gabriele Di Giacomo1, Luca Taglieri1, Francesca Scimia1, Antonio Trifirò2, Emanuela Cocconi2, Enrique Martínez de la Ossa3, Clara Pereyra3
1Department of Industrial Engineering, University of L’Aquila, L’Aquila, Italy
2SSICA-Stazione Sperimentale per l’Industria delle Conserve Alimentari, Parma, Italy
3Department of Chemical Engineering, University of Cadiz, Cadiz, Spain
Correspondence to: Gabriele Di Giacomo, Department of Industrial Engineering, University of L’Aquila, L’Aquila, Italy.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The inactivation of Pectin Methylesterase (PME), and Peroxidase (POD), in fresh single-strength carrot juice by supercritical carbon dioxide (SCCD) at 40 °C and a CO2/juice ratio (w/w) equal to 3 was investigated. In the range of pressure from 10 to 25 MPa and treatment time from 40 to 110 min, both PME and POD were effectively inactivated significantly. The laboratory inactivation tests were performed by using a semi continuous method with a continuous stream of CO2 fed at constant mass flow rate from the beginning to the end of each experimental test. A semi empirical kinetic model that accounts for a residual equilibrium fraction, 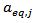 , of POD and PME was used to describe satisfactorily the inactivation behavior. Under the above mentioned experimental conditions the pressure dependence of the kinetic constants,
, of POD and PME was used to describe satisfactorily the inactivation behavior. Under the above mentioned experimental conditions the pressure dependence of the kinetic constants, 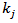 , can be described satisfactorily by a constitutive equation that is a linear function of the reduced pressure,
, can be described satisfactorily by a constitutive equation that is a linear function of the reduced pressure, 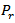 , of carbon dioxide. The numerical values of the two model parameters, calculated by fitting the experimental data in terms of dimensionless residual activity measured at the end of each experimental tests are: 0.04035 min-1, 0.10720 for POD and 0.00345min-1, 0.08941 for PME.
, of carbon dioxide. The numerical values of the two model parameters, calculated by fitting the experimental data in terms of dimensionless residual activity measured at the end of each experimental tests are: 0.04035 min-1, 0.10720 for POD and 0.00345min-1, 0.08941 for PME.
Keywords: Carrot juice, Supercritical CO2, Enzymes inactivation, Kinetic constants, Residual activity
Cite this paper: Gabriele Di Giacomo, Luca Taglieri, Francesca Scimia, Antonio Trifirò, Emanuela Cocconi, Enrique Martínez de la Ossa, Clara Pereyra, Effect of Supercritical Carbon Dioxide on the Enzymes Inactivation in Single-Strength Carrot Juice, International Journal of Food Science and Nutrition Engineering, Vol. 4 No. 4, 2014, pp. 106-111. doi: 10.5923/j.food.20140404.03.
Article Outline
1. Introduction
- Carrot is one of the top-ten most economically important vegetables crops widely cultivated in many parts of the world [1]. Currently, China is the first producer with almost 50% of the global world production. About 60% of world carrots production occurred in Asia, followed by the Europe (20%), Americas (10%), and Africa (4%). From the early 70s to today, the global world production of carrots increased about five times, significantly more than the overall increases in world vegetable production [2]. Carrots are an excellent source of vitamins, dietary fibers, minerals and other phytonutrients like polyacetylenes able to interact with carotenoids in preventing the growth of colon cancer cells and coronary hearth disease [3, 4]. These properties make carrots of particular interest as a source of juice prepared ready to drink and stimulate the industrial production of enhanced shelf-life (ESL) carrot juice (CJ) of comparable quality. However, since CJ is thermal sensitive, the application of a number of non-thermal preservation technologies have been investigated during the last two decades as substitute of the thermal treatment [5-10]. Among others, the one based on the use of gaseous-high-density carbon dioxide, usually in the supercritical state, (DPCD) appears to be very promising for processing liquid food and juices of fruits and/or vegetables, including fresh CJ [10]. In fact, it can be applied at the temperature of human and animal body, while typical operating pressure values are thirty times smaller than those typical of the treatment at high hydrostatic pressures (HHP). In general, DPCD has potential advantages over other non-thermal processes in terms of both capital and operating costs since it is easy to scale-up to continuous industrial processing operating with recirculating CO2, thereby avoiding significant loss of the processing fluid and flavours. One of the main objectives in the industrial processing of CJ is to stabilize the cloudiness of the product during storage, while preserving quality parameters such as colour, flavour, viscosity and nutritional characteristics. In this respect, is well recognized, for example, that pectic enzymes and in particular pectin methylesterase (PME) influence viscosity and cloud stability, while peroxidase (POD), characterized by high thermo-stability, is involved in the oxidation of many organic compounds, leading to deterioration in flavour, colour, nutritional and functional properties [9-11]. Therefore, the inactivation of indigenous PME and POD in fruits, vegetables and derived beverages has been widely investigated but, in many case, this has been done using model aqueous solutions neglecting, in this way, the effect of decreasing pH and interaction with other substances present in the natural matrix [12-14]. The purpose of this work is to quantify and to simulate the capability of DPCD in inactivating PME and POD in fresh, single-strength CJ obtained from fresh carrots.
2. Materials and Methods
2.1. Materials
- PGI (protected geographical indication) carrots (Daucus Carota L.) officially named “Carotadell’ Altopiano del Fucino” were taken from a local farmer that also operated a large carrots and vegetables juices industrial plant. Carrots were stored at 2°C for a time never longer then three days until used for producing the amount of juice required for making a laboratory experimental test. To this purpose, after washing with chlorinated water, rinsing with tap water, and peeling, the juice was obtained using a juice extractor (MULINEX FRUTTI-PRO XL). Then, the juice having a pH about 6 was filtered through a 4-lyers cheesecloth, divided in three parts and suddenly used for analysis and for processing. The third portion was bottled, sealed and stored in a refrigerator at about 2 °C to be used as reference sample in the evaluation of ESL.
2.2. Enzyme Assay
- Peroxidase activity was assayed by the Pyrogallol test [15] with minor modifications, at pH=6 and 20 °C, using 20 g of homogenized sample previously filtered on filter paper. Pectin Methylesterase activity was assayed by evaluating the carboxylic groups that are released as a result of the reaction of the indigenous PME on added Pectin (P9135 Sigma). Titration occurs automatically (pH-Stat option, Metrohm) by recording the amount of 0.1 N NaOH supplied over time, which is necessary to maintain pH at 7.5, at 30 °C. 10 g of homogenized sample previously filtered on filter paper mixed with 200 mL of NaCl solution and adjusted to pH = 7.5 with 0.1 N NaOH, were used.
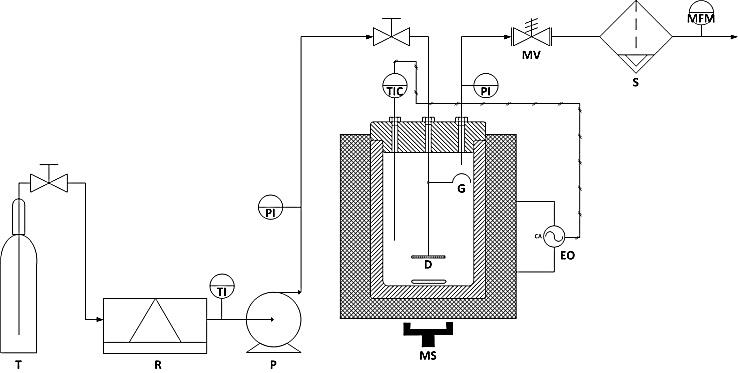
2.3. Experimental Apparatus and Procedure
- The main component of the system (Figure 1) is a 0.250 L AISI 329 stainless still autoclave (NOVA-WERKE, Zurich, Switzerland) designed to operate up to 70 MPa at 773 K with three holes on the head which could be removed before the beginning and at the end of each experimental test. The reactor was equipped with a variable speed magnetic stirring system and a gas diffuser to achieve an intimate contact between the liquid inside the vessel and the CO2. A membrane high-pressure metering pump (flow rate: 0-3 kg/h; Pmax = 35 Mpa; MILTON ROY) was used for feeding the reactor with liquid CO2 at a predetermined constant rate, continuously monitored by a mass-flow-rate (MICROMOTION, D6) located at the exit of the expansion vessel. The desired operating temperature was obtained by inserting the autoclave into an electrical oven equipped with two heating resistances (Wallow, MT03L3AP-3000, 200 V, 1000 W) and an automatic temperature control system (CAL Controls, 3300). A type J thermocouple connected to one of the three holes available on the head with an high-pressure pipe having an internal diameter of 0.001 m, was inserted into the reactor in order to continuously detect the temperature inside the cell with an accuracy of ± 0.2 K, while the internal pressure was continuously monitored by a pressure transducer (HAENNI, EDR430), fitted on the reactor head, with an accuracy of 0.15%. To start a run, the cleaned reactor was hermetically closed, heated at the desired temperature, evacuated and filled with 0.190-0.220 L of CJ taken from the refrigerator. Then, the pump of the inlet CO2 liquid stream was operated to pressurize the cell; when the predetermined pressure value was reached, the high-pressure metering valve located on the exit line of CO2 was opened in order to keep a constant CO2/CJ = 3 (w/w) with the help of the above mentioned mass flow-meter. At the end of each run the reactor was slowly depressurized and suddenly opened to recover the treated CJ that was collected in two sterile glass bottles, refrigerated at 2 °C and then analysed.
2.4. Kinetics of Enzymes Inactivation
- It is reasonable to assume that at 40 °C the kinetics of enzymes inactivation is not affected by the temperature [16] at least for the time required for the treatment with supercritical CO2 (SCCD), while it is reasonable to expect that the degree of inactivation is affected by the treatment time and by the value of the operating reduced pressure of CO2. Furthermore, the presence of a labile and a stable fraction of the enzymes exposed to different inactivation treatments was observed and reported in the literature along with convincing argumentations for a number of fruits, vegetables, and derived products including CJ, and for a number of enzymes, including POD and PME [11-13]. The following semi empirical model, eq. 1, for the kinetics of inactivation of indigenous POD and PME in single strength CJ, accounts for all the above mentioned considerations.
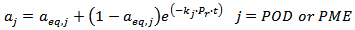 | (1) |
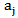 is the dimensionless residual activity of POD or PME defined as
is the dimensionless residual activity of POD or PME defined as 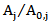 ;
;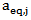 is the dimensionless residual activity of POD or PME after prolonged treatment (equilibrium conditions) defined as
is the dimensionless residual activity of POD or PME after prolonged treatment (equilibrium conditions) defined as 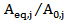 ;
;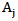 is the residual activity of POD or PME (Units/g) after t min of treatment;
is the residual activity of POD or PME (Units/g) after t min of treatment;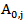 is the activity of POD or PME (Units/g) in the untreated single strength carrot juice;
is the activity of POD or PME (Units/g) in the untreated single strength carrot juice;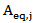 is the residual activity of POD or PME (Units/g) after prolonged treatment (equilibrium conditions);
is the residual activity of POD or PME (Units/g) after prolonged treatment (equilibrium conditions);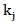 is the kinetics constant (min-1) of POD or PME at 40 °C and CO2/CJ = 3 (w/w);
is the kinetics constant (min-1) of POD or PME at 40 °C and CO2/CJ = 3 (w/w);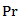 is the operating reduced pressure of SCCD.Equation 1 was obtained by the fundamental equation representing the POD or PME conservation law in combination with: a) a first-order fractional kinetic model for enzymes inactivation; b) a linear dependence with the pressure of the kinetic constant at 40 °C and a CO2/CJ = 3 (w/w). More particularly the following plain constitutive equation:
is the operating reduced pressure of SCCD.Equation 1 was obtained by the fundamental equation representing the POD or PME conservation law in combination with: a) a first-order fractional kinetic model for enzymes inactivation; b) a linear dependence with the pressure of the kinetic constant at 40 °C and a CO2/CJ = 3 (w/w). More particularly the following plain constitutive equation: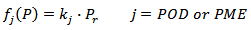 | (2) |
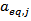 and the
and the 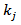 of POD or PME. It is worth to underline that, while
of POD or PME. It is worth to underline that, while 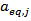 is a single value (one for POD and one for PME),
is a single value (one for POD and one for PME), 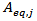 (Units/g) is proportional to
(Units/g) is proportional to 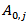 (Units/g), which is related to the carrots used to prepare the juice in terms of variety, seasonality, duration and mode of storage, manufacturing technology of the juice, and others.
(Units/g), which is related to the carrots used to prepare the juice in terms of variety, seasonality, duration and mode of storage, manufacturing technology of the juice, and others.2.5. Statistical Analysis
- To validate eq. 1, the values of the Percent Average Absolute Error (PAAE) defined by:
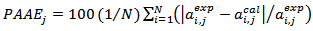 | (3) |
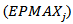 and the coefficients of determination
and the coefficients of determination 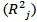 were determined for
were determined for 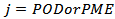 . Moreover, for validation of eq. 1, the bias factors
. Moreover, for validation of eq. 1, the bias factors 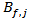 and the accuracy factors
and the accuracy factors 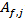 were also calculated to compare the experimental values
were also calculated to compare the experimental values 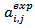 with the predicted values
with the predicted values 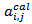 given by the model.
given by the model. 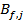 and
and 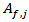 [18, 19] were calculated by eqs. 4 and 5, respectively.
[18, 19] were calculated by eqs. 4 and 5, respectively.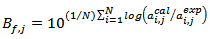 | (4) |
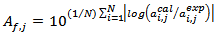 | (5) |
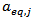 and
and 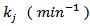 were calculated along with their significance at 95% confidence, by fitting eq. 1 to the experimental data,
were calculated along with their significance at 95% confidence, by fitting eq. 1 to the experimental data, 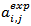 .
.3. Results
- The following least square function was minimized to parameterize the kinetic model (eq. 1).
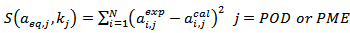 | (6) |
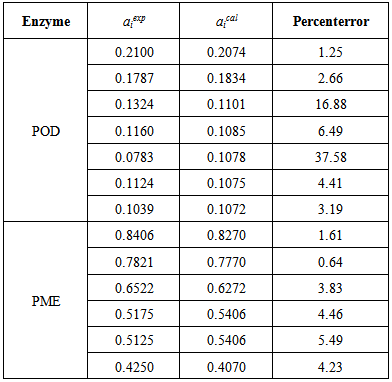
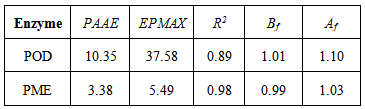
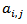 for POD and PME were reported in Table 2 along with the corresponding experimental values and with the corresponding percent errors. All the statistical parameters described in the previous section were also calculated and reported in Table 3 to quantify the performance of the kinetic model. As can be seen the results are satisfactory for both POD and PME. Furthermore, Figure 2 shows graphically that the calculated values of
for POD and PME were reported in Table 2 along with the corresponding experimental values and with the corresponding percent errors. All the statistical parameters described in the previous section were also calculated and reported in Table 3 to quantify the performance of the kinetic model. As can be seen the results are satisfactory for both POD and PME. Furthermore, Figure 2 shows graphically that the calculated values of 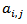 compare reasonably well with the corresponding experimental values in the whole range of the experimental operating parameters. Finally, to better show the effect of the pressure of the supercritical carbon dioxide stream and of the treatment time on the POD an PME inactivation, the calculated behaviour of
compare reasonably well with the corresponding experimental values in the whole range of the experimental operating parameters. Finally, to better show the effect of the pressure of the supercritical carbon dioxide stream and of the treatment time on the POD an PME inactivation, the calculated behaviour of 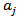 were reported in Figures 3 and 4, along with the experimental data. As can be seen a reduction greater than 90% can be obtained for the enzymatic activity of POD and of about 70% for PME.
were reported in Figures 3 and 4, along with the experimental data. As can be seen a reduction greater than 90% can be obtained for the enzymatic activity of POD and of about 70% for PME.
|
|
|
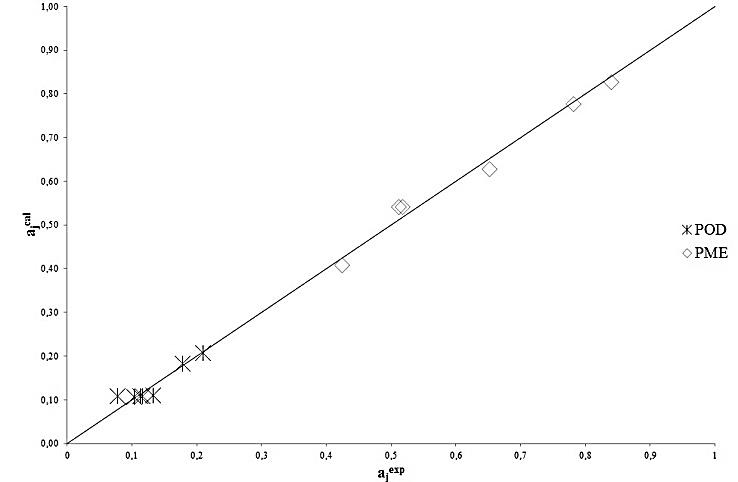 | Figure 2. Experimental versus calculated values of the dimensionless residual activity, ai, of POD and PME |
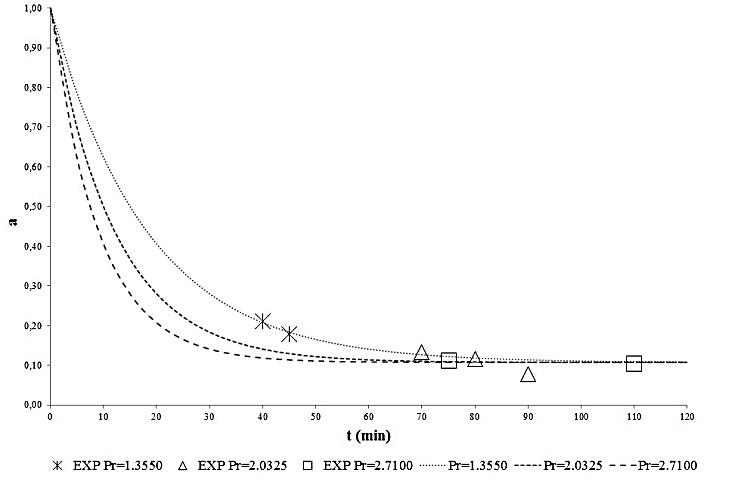 | Figure 3. Effect of supercritical CO2 stream pressure and duration of treatment on POD inactivation |
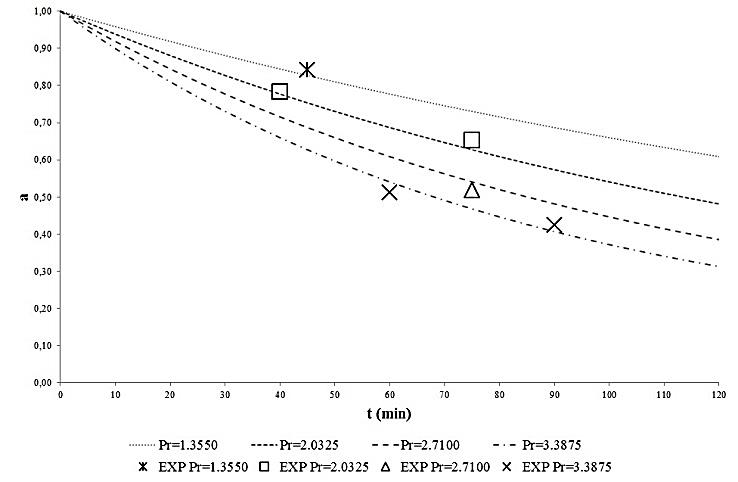 | Figure 4. Effect of supercritical CO2 stream pressure and duration of treatment on PME inactivation |
4. Conclusions
- The activity of indigenous POD and PME in fresh single-strength carrot juice can be significantly reduced by applying DPCD treatments. The experimental results obtained with a semi-continuous laboratory apparatus operated at 40 °C, CO2/CJ = 3 (w/w), pressures from 10 to 25 MPa, and treatment time from 40 to 110 min show that the labile fraction of the POD activity was completely inactivated, while the labile fraction of the PME activity was reduced of about 80%. The enzymes activity decreases with increasing CO2 stream pressure and treatment time; this behaviour is quantitatively described by a semi-empirical, fractional, first-order kinetic model. Further work is required to optimize the operating process parameters using a continuous pilot plant with recirculating CO2.
ACKNOWLEDGEMENTS
- This study was financially supported by the national ministry of the university and research (MIUR). We thank Mr. G. Spagnoli for the daily help given in the laboratory to make the experimental tests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML