-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2014; 4(4): 98-105
doi:10.5923/j.food.20140404.02
Storage and Drying Time Effects on Digestibility and Amino Acid Composition of Dried Smoked Horse Mackerel (Trachurus trachurus) Fillets
Atowa C. O.1, Nwabu A. O.1, Ogiedu T. A.2
1Department of Food Science and Technology, University of Nigeria, Nsukka, Nigeria
2Department of Food Technology, Auchi Polytechnic, Auchi, Nigeria
Correspondence to: Atowa C. O., Department of Food Science and Technology, University of Nigeria, Nsukka, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study assessed the effect of storage on the amino acid composition of dried horse mackerel fillets. Dried horse mackerel (Trachurus trachurus) fillets were prepared by smoking brined horse mackerel fillets in a traditional improved kiln for 4 hours at 60-70℃. The fillets were later oven-dried at 65-70℃ for 0, 2, 4, 6, and 8 hours. There was a significant difference (p < 0.05) in-vitro protein digestibility (IVPD) of dried fillets over the 8 hours of drying (from 99.44% in control sample to 95.02% for the 8 hour dried sample), while storage decreased the IVPD of the samples from a range of 99.44 - 94.07% in control sample to 95.02 to 89.60 % in the 8 hour dried sample. Drying reduced the amino acid composition of the horse mackerel fillets from 47.10 g/100g protein in control sample to 27.14 g/100g protein in 8 hour dried. The results also showed that the total amino acids significantly reduced (p < 0.05) from the range of 47.10 to 27.14 g/100g protein on the 0 day to 27.42 -14.58 g/100g protein on the last (30th) day of storage. Essential amino acids reduced from the range of 25.40 - 14.01 g/100g protein on the 0 day to 14.64 - 7.42 g/100g protein while the non essential amino acids also decreased from a range of 21.71 - 13.13 g/100g protein on the 0 day to 12.78 - 7.16 g/100g protein on the last day of storage. In conclusion, both drying and storage affected the IVPD and the amino acid composition. However, results showed that they could still meet the amino acid requirement of an adult (70 kg) man.
Keywords: Fish, Drying, Amino acid composition, Protein quality, In-vitro protein digestibility
Cite this paper: Atowa C. O., Nwabu A. O., Ogiedu T. A., Storage and Drying Time Effects on Digestibility and Amino Acid Composition of Dried Smoked Horse Mackerel (Trachurus trachurus) Fillets, International Journal of Food Science and Nutrition Engineering, Vol. 4 No. 4, 2014, pp. 98-105. doi: 10.5923/j.food.20140404.02.
Article Outline
1. Introduction
- Fish drying is one of the most important traditional fish preservation methods in tropical countries like Nigeria, where they make use of the high ambient temperature and low humidity. Its main importance is in reducing the moisture content of the flesh to a limit that ensures the stability of the product in storage for a longer period of time. Traditionally, dried fish represents a low cost source of high quality protein [1]. With the improvement in indigenous technology, local dryers have been developed to complement sun drying in most communities, which enhances dried fish quality due to its ability to protect fish from dust, infestation and contamination. However, some fish mongers especially those within the rural communities, still practice a simple and inexpensive fish drying process to produce a dried fish. Though commercially accepted, fish processed through this means are exposed for longer heat period for effective drying which leads to severe nutritive losses [2] due to complex chemical reactions, such as protein-protein interactions, protein-fat interaction [3, 4] as a result of high processing temperatures. With increased awareness on the health benefits of fish consumption, fish consumption is on the high side. They are consumed as smoked, dried, fried, boiled or canned fish. Smoke-dried fish products are consumed more than fish prepared through any other means. They are favoured because the smoke-drying process imparts characteristic aroma, taste, colour and texture (crispiness) on processed fish [5]. Dried fish are less expensive than fish prepared through any other means. The product is widely consumed as there is no religious or cultural objection to its consumption.According to [6], the preservation and processing of fresh water and marine fishes largely depend on the technology developed in any particular area. Due to erratic power supply and the unavailability of storage facilities for most rural households, dried fish which store for several weeks before consumption represents the most inexpensive source of animal protein for rural dwellers. As a result of high temperature involved in fish drying and the long storage weeks, most of the proteins are denatured and this leads to reduction in functionality of essential amino acids. The totality of these, often results in fish products with low nutritive value, thereby exposing the populace who rely on dried fish products for their source of animal protein nutritionally insecure. This work, therefore, was aimed at evaluating the effect of storage time on the digestibility and amino acid composition of dried horse mackerel fillets over different drying time.
2. Materials and Methods
2.1. Materials Procurement
- Fifty pieces (15.2 kg) of frozen horse mackerel (Trachurus trachurus) were purchased from cold at Nsukka; salt for brining were obtained from Nsukka main market, while the wood and saw dust for smoking were obtained from timber shed Nsukka, Enugu state.
2.2. Preparation of Smoke-dried Fish
- The fish samples were transported under ice to the fish processing unit, University of Nigeria, Nsukka; allowed to thaw at room temperature for 45 minutes, washed with tap water and weighed using weighing balance (OQUISM, Hamburg Quality Manufacturing Equipment Germany). Samples were eviscerated, filleted, washed and loss in weight determined. The fish fillets (7.4 kg) was brined for 30 seconds in 75° saturated brine, rinsed in fresh water, spread on perforated trays and kept for 30 minutes to drain. Thereafter the trays were transferred to a traditionally improved oven for smoking. The firewood was lighted and allowed to burn briefly before moist saw dust was applied to smoulder the fire. The temperature of the smoking chamber was maintained (70 – 80℃ for 4 hours) by adjusting the firewood and sawdust in the hearth, after which, it was cooled overnight. The fish were then taken to a conventional oven (VWR1370, Sheldon Manufacturing, Cornelius Oregon USA) for drying. The smoked fish fillets were dried in a drying oven at a temperature of 70 – 80℃ to reduce the moisture content and consequently toughen the fillets. The drying treatment was carried out for varying durations of 0 (control sample), 2, 4, 6, and 8 hours. All the batches were spread on separate trays at room temperature for a period of 30 days during which the amino acid composition and in-vitro protein digestibility (IVPD) of all the samples were monitored after every 10 days.
2.3. Determination of Amino Acid Content
- Amino acid determination was done in accordance with the Amino Acid Sequential Technico method. About 20 µg of the fish samples were dried in conventional hydrolysis tubes. To each tube 100 µL of 6 mol L-1 HCl containing 30 ml phenol and 10 ml 2-mercaptoethanol (6 mol L-1 HPME) were added and the tubes were evacuated, sealed and hydrolysed at 110℃ for 22 hours. After hydrolysis, HCl was evaporated in a vacuum bottle heated to about 60℃. the residue was dissolved in a sample buffer and analysed for amino acids using RP-HPLC with an Agilent 1100 assembly system (Agilent Technologies, Palo Alto, CA 94306, USA) and Zorbax 80A C18 column (4.6 id × 180 mm). The Excitation Wavelength (Ex) of 348 nm and Emission Wavelength (Em) of 450 nm were chosen. The column oven was maintained at 60℃. Amounts of amino acids were determined by calculations using the recorded chromatogram. Determination of tryptophan was done by the Ninhydrin method. One gram of each sample was put into a 25 ml polypylene test tube with caps, 10 ml of 0.075N NaOH was added and thoroughly mixed until clear solution was obtained. The dispersion was shaken for 30 minutes and centrifuged at 5000 rpm for 10 minutes and the supernatant liquid transferred into a clean test tube. About 0.5 mL of the supernatants, 5 ml of ninhydrin reagent (1.0 g of ninhydrin in 100 ml mixture of 37% HCl and 96% HCOOH) in a ratio 2:3 for all the samples were added and incubated at 35℃ for 2 hours. After incubation, the solution was cooled to room temperature (23 - 25℃) and the volumes were made up to 10 ml using diethyl ether, thoroughly mixed using vortex mixer, filtered and the clear filtrates were analysed with the same equipment as described above for the other amino acids. Amino acid was expressed as g/100g of proteins.
2.4. Determination of in Vitro Protein Digestibility
- The in-vitro protein digestibility was determined by the method described by [7]. Pepsin followed by pancreatic digest was prepared by incubating 100 mg of protein equivalent sample with 1.5 mg pepsin in 15 ml of 0.1N HCl at 37℃ for 3 hours. After neutralization with 0.2N NaOH, 4 mg pancreatic in 7.5 ml phosphate buffer of pH 8.0 was added. Just 1 ml of toluene was subsequently added to prevent microbial growth and the solution incubated for additional 24 hours at 37℃; the nitrogen content in the solution after 24 hours of digestion was taken as a measure of the digested product. Following the 24 hour incubation, the enzyme was inactivated by the addition of 10 ml of 10% trichloroacetic acid (TCA) to precipitate undigested protein, which was filtered off. The volume of the filtrate was made up to 100 ml and centrifuged at 5000 rpm for 20 minutes; the supernatant was collected for N determination by the method of AOAC [8]. Reagent/enzyme blanks was also digested alongside the sample under the described conditions and by using 1 g of each source of enzyme to make N measurement easier.The digestibility of the protein was calculated using the equation below
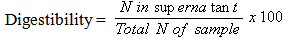
3. Results and Discussion
3.1. In-vitro Protein Digestibility
- The in vitro protein digestibility of smoked and dried horse mackerel fillets is presented in Figure 1. The highest protein digestibility was observed in control sample (99.44 %). There was no significant difference (p > 0.05) between the control and the sample dried for 2 hours. Heat treatment positively influenced the digestibility of proteins, all the freshly dried samples in this study recorded higher value of IVPD compared with that of raw mackerel fish (93 %) as reported by [9]. Protein digestibility is influenced by the presence of antinutritional factors [4], different processing methods that affect the levels of those antinutritional factors, will consequently influence protein digestibility.
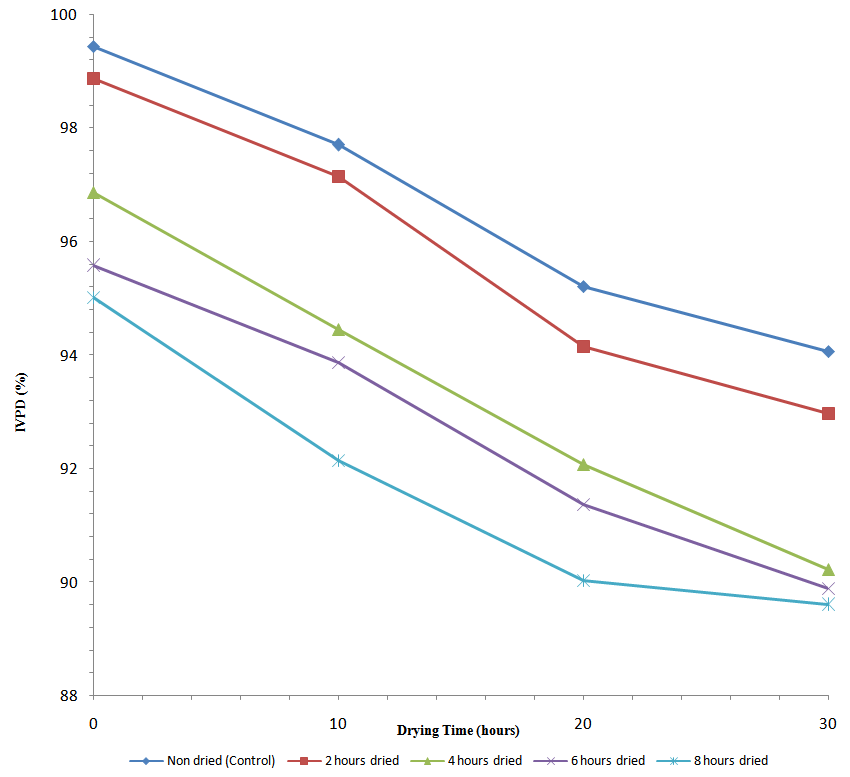 | Figure 1. Changes in in-vitro protein digestibility (IVPD) (%) of smoked and dried horse mackerel fillets during storage |
3.2. Amino Acid Composition
- The amino acid composition of smoked and dried horse mackerel fillets is shown in Table 1-4. The amino acids were classified as essential and non essential. The essential amino acids considered in this study were phenylalanine, tyrosine, leucine, lysine, methionine, tryptohan, valine, threonine, and histidine while the non essential amino acids were alanine, glutamine, glutamic acid, glycine, serine, arginine, asparagine and trimethylysine. The dominant essential amino acids were found to be tryrosine, methionine, leucine and lysine. [12] reported methionine, threonine, isoleucine, leucine, phenylalanine and valine to be dominant in grilled and fried horse mackerel. [10] observed lysine and leucine as dominant amino acids in Persian sturgeon fish. The dominant non essential amino acids in this study were alanine, glycine and proline. [10] reported dominant non essential amino acids in Persian sturgeon to be glutamic acid. Tyrosine showed the highest concentration of all amino acids in the smoked fish sample (which served as control). [13, 2], [14, 10, 12] reported that smoking improves the amino acid composition of fish. The amino acid composition of dried horse mackerel fillets in this study ranged between 47.10 g/100g protein for (non-dried smoked fish to 27.14 g/100g protein for 8 hour dried horse mackerel on the 0 day of storage.Table 1-4 showed that the samples had higher concentration of essential amino acids than the non-essential amino acids. The essential amino acids ranged from 25.40 g/100g protein to 14.01 g/100g protein, significantly lower than 33.9 g WHO standard protein. The reduction could be due to destruction as a result of heat treatment [4, 2]. The non essential amino acids ranged from 21.70 g/100g protein to 13.13 g/100g protein on freshly dried samples. [2] reported higher amount of essential amino acids in canned fish samples (33.4 to 48 g/100g protein) than WHO standard protein (32g/100g protein). [10] observed the range of essential amino acids in grilled and fried fillets to be 42.70 to 42.9 g/100g protein. [10], also observed that grilling increased the essential amino acids while frying slightly decreased it. The significantly (p < 0.05) lower values observed in this study may not be unconnected with the severe treatment administered to this fish (brining, smoking and drying) [3], small sizes of the fish, season and environmental influence [15], and the effect of long cold storage that the fish were subjected to during transportation and distribution [9]. Another fact could also be result of interactive actions of smoke components on the amino acids [6].
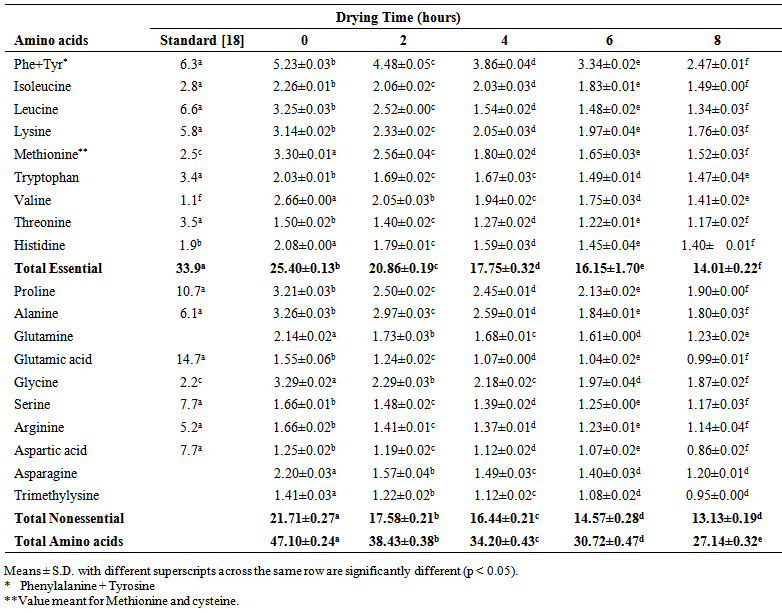 | Table 1. Amino acid composition (g/100g protein) of smoked and dried horse mackerel fillets during storage (Day 0) |
 | Table 2. Amino acid composition (g/100g protein) of smoked and dried horse mackerel fillets during storage (Day 10) |
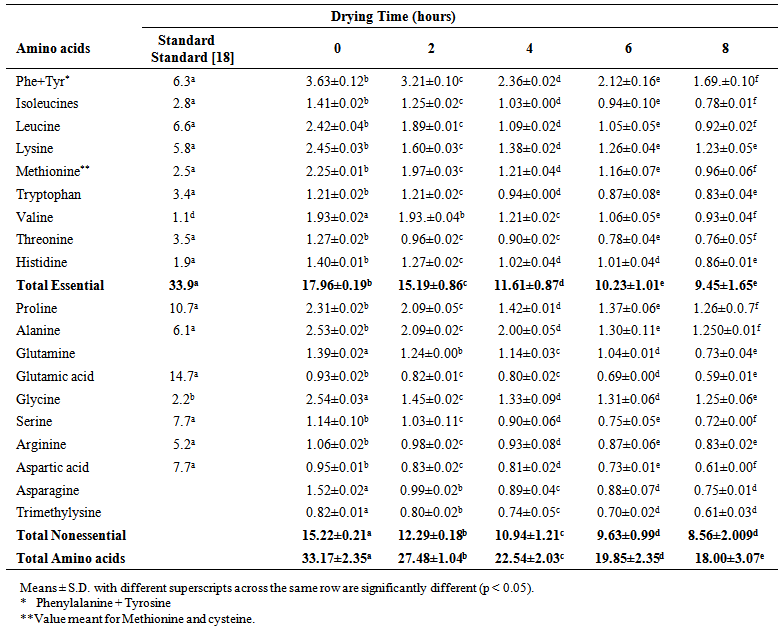 | Table 3. Amino acid composition (g/100g protein) of smoked and dried horse mackerel fillets during storage (Day 20) |
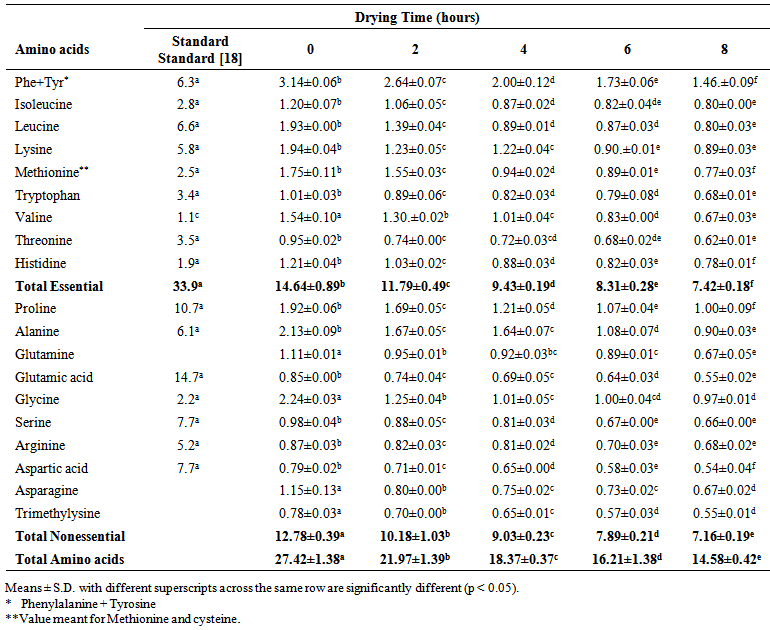 | Table 4. Amino acid composition (g/100g protein) of smoked and dried horse mackerel fillets during storage (Day 30) |
4. Conclusions
- The study revealed that longer drying time affects the amino acid composition of dried fish products, the protein were not hydrolysed as storage proceeded thereby reducing the protein quantity and quality levels of dried horse mackerel products during storage. However, it was noted that even though the drying reduced the amounts of amino acids present below that of standard protein dried fish products can still meet the recommended essential amino requirement of an adult. Also, amino acids in dried fish is still higher than that in cereals and so can still be used to supplement the amino acid profile of cereal dependent diets. Treatment effects on protein digestibility of the dried fish were not much felt with drying as amino acids. Hence, it seems probable that although amino acids may be rendered unavailable when fish are subjected to heat treatment (60-70 oC), that a rather severe heating is required to render the fish protein resistant to the action of digestive enzyme. Nevertheless, longer drying and/or storage time could render the amino acid quality of dried fish below the recommended dietary allowance. It can also be concluded that both treatment and storage had a negative influence on the protein digestibility in this study.
ACKNOWLEDGEMNTS
- The authors are grateful to the technicians of the Institute of Tropical Agriculture Ibadan and to all those who contributed in one way or another to successfully conclusion of this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML