-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2014; 4(3): 80-85
doi:10.5923/j.food.20140403.04
Extraction and Partial Kinetic Properties of Invertase from Schizosaccharomyces pombe
Amna A. A. Aburigal1, Elamin A. Elkhalifa1, Abdel Moneim E. Sulieman2, Hassan B. Elamin3
1Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira
2Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
3Commission for Biotechnology & Genetic Engineering, National Centre for Research, Khartoum, Sudan
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Invertase enzyme has a wide range of applications in food industry. The aim of this study was to extract invertase from the yeast Schizosaccharomyces pombe namely; strains H andL. Strains were isolated from fermented honey Duma. Spectrophotometric methods were used to study enzyme kinetics and to determine the factors affecting enzyme activity. Invertase extracted from Schizosaccharomyces pombe H exhibited the maximum activity at pH 4.5 and temperature of 80℃, and retained about 15% of its activity after 35 minutes of incubation at 80℃. Whereas invertase from Schizosaccharomyces pombe L showed the maximum activity at pH 4.5 and temperature 60℃, and was quite stable and retained 35% of its maximum activity at 60℃ after 35 minutes of incubation. Maximum activity was also indicated at sucrose concentrations of 1.9M and 1.2M, respectively. Vmax and Km were found to be 70.27mM/min and 20.67mM for invertase from S.pombe H and 58.24mM/min and 11.36mM for invertase from S.pombe L, respectively. The extracted invertase from S.pombe could have a potential to be used in food processing.
Keywords: Invertase, Schizosaccharomyces pombe, Duma, Spectrophotometric method, Enzyme stability, Thermal activity, Stability
Cite this paper: Amna A. A. Aburigal, Elamin A. Elkhalifa, Abdel Moneim E. Sulieman, Hassan B. Elamin, Extraction and Partial Kinetic Properties of Invertase from Schizosaccharomyces pombe, International Journal of Food Science and Nutrition Engineering, Vol. 4 No. 3, 2014, pp. 80-85. doi: 10.5923/j.food.20140403.04.
Article Outline
1. Introduction
- Enzymes are biomolecules synthesized by the cell in order to bring about certain biochemical reaction under mild conditions. They act on substrates specifically and ultimately cause transformation or conversion of substrates to products. Enzymes are considered industrially and commercially important biomolecules, because they have several advantages over inorganic catalysts including high specificity, high rate of reaction, non toxicity, water solubility, reproducibility under normal laboratory conditions, mild conditions of pH and temperatures [1].Utilization of microbial sources material for enzyme production has increased steadily for the following reasons: the high specific activity, seasonal fluctuation of raw material and possible shortage. There is a wide spectrum of characteristics such as optimum pH and temperature resistance available for selection and there are possibilities of greatly optimizing enzyme yield through strain selection and growth conditions. There are several important factors, which may affect the rate of enzyme-catalyzed reactions. The most important among them are the enzyme and substrate concentrations, temperature and pH [2].A commercially important enzyme in food processing is invertase, known as β-fructofuranocidase (E.C.3.2.1.26). There are many industrial applications of invertase in confectionary, beverage, bakery and pharmaceutical formulations. It plays a role in the production of invert syrup i.e., equimolar mixture of fructose and glucose from sucrose. Invert syrup produced by enzyme hydrolysis is preferred over more than those syrups produced by acid hydrolysis of sucrose giving undesirable by- products, bitter after taste; low conversion efficiencies, high ash contents and so are highly uneconomical. On the other hand, the enzymatic hydrolysis gives high purity products that have an acceptable taste, stability, non-crystalizable and free from any undesirable by-products [3-9]. A wide variety of microbial, animals or plant sources are used for the production of industrial enzymes. But most enzyme production processes rely on the microbial source. Invertases from Saccharomyces cerevisiae are usually active in slightly acidic to neutral pH (3.0-7.0), to close to neutral pH (6.5-7.0), and in a temperature range of roughly 30℃ to 75℃ [10]. Belcarz et al. [11] reported that invertases produced by Candida utilis showed high tolerance to temperature changes and were active from 30-90℃. However, optimum activity was obtained at 70℃. The enzymes were highly active at pH 3.6 to 5.0 with optimum pH 4.5. Nguyen et al., [12] found that Aspergillus niger invertase was stable at pH range from 5.0 to 6.5 and temperature up to 60℃. The objectives of present work were to extract invertase enzyme from yeast Schizosaccharomyces pombe isolated from mead fermented honey (Duma) and to determine its kinetics properties.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
- The yeast Schizosaccharomyces pombe strains H and L were used for invertase production by shake flask technique. Yeast culture was maintained on the medium containing sucrose 20.0 g/L; agar 20.0 g/L; peptone 5.0 g/L; malt extract 3.0 g/L and yeast extract 3.0 g/L at pH 6.0 [13].
2.2. Preparation of Vegetative Inoculums
- To prepare the vegetative inoculums, 25 ml of medium was transferred to each 250 ml Erlenmeyer flask. The flasks consisted of sucrose 30.0 g/L; peptone 5.0 g/L; malt extract 3.0 g/L and yeast extract 3.0 g/L at pH 6.0. The flasks were cotton plugged and autoclaved at 103.5 Pa pressure, 121℃ for 15 minutes and cooled at room temperature. One ml of inoculums was transferred to each flask under sterile conditions. Flasks were then incubated in a rotary incubator shaker at 30℃ for 24 hours. Agitation rate was kept at 200 rpm [13]. The cultures were stored at 4℃. Yeast cells were obtained by centrifugation of fermented broth at 5000 rpm.
2.3. Extraction of Yeast Invertase
- For extraction of invertase from the yeast, 5 g of yeast cells were suspended in deionized water (1:1 w/w). Toulene (3%) and sodium carbonate (1%) were added. The suspension was incubated for 4 days at room temperature allowing cell autolysis to occur. The crude extract was centrifuged at 4000 rpm at 4℃. The pH in supernatant was adjusted to 4.0 with 1M sulphuric acid and allowed to sediment overnight at 4℃. The sediment was removed by centrifugation as above. The extracted invertase was precipitated at 4℃ by addition of cold 96% ethanol to 50% (v/v) saturation. The pellets which contained invertase were completely dissolved in deionized water and dialyzed over night at 4℃ against deionized water. Material precipitated during dialysis was removed by centrifugation. The obtained supernatant represented the extract [14].
2.4. Enzyme Activity
- The conditions at which the enzyme showed best performance were determined. The information would be helpful in food application to obtain an appreciable efficiency. In order to understand the enzyme kinetics, it is important to understand maximum velocity (Vmax) and Michaelis-Menten constant (Km). The rate of reaction catalyzed by an enzyme increases linearly with the substrate concentration up to a point, but it soon reaches the maximum value called Vmax beyond which there is no further increase in reaction rate. Michaelis and Menten defined a constant designated as Km, which is useful in establishing the accurate relationship between the substrate concentration and the velocity of the enzyme – catalyzed reaction [15]. Effect of temperature, heat stability, pH, substrate concentration, km, Vmax and enzyme inhibitors were determined.
2.5. Enzyme Activity Assay
- β-Fructosidase hydrolyses α 1,2 glycosidic bonds in sucrose and other oligoglycosides. If the sample contains sucrose only, it is measured specifically via D- glucose [16]. Glucose formation in the samples was measured by Glucose - Oxidase method which was done with Glucose Hexokinase (HK) Assay Kit (Biosystem, Spain, 2010). Glucose assay kit was used for the quantitative enzymatic detection of glucose in solutions. The kit , which contains 1.5 mM NADP, 1.0 mM ATP, 1.0 unit/ml of hexokinase, and 1.0 unit/ml of glucose-6-phosphate dehydrogenase with sodium benzoate and potassium sorbate as preservatives in 20ml distilled water, utilizes two enzyme, hexokinase and glucose-6- phosphate dehydrogenase.
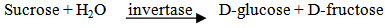 | (3.1) |
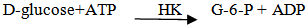 | (3.2) |
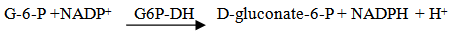 | (3.3) |
2.6. Thermal Enzyme Activity
- In order to determine the effect of temperature on the activity of the enzyme, activity assays were carried out over the range 30-80℃, using sucrose as a substrate. A 50g/L sucrose solution, buffered with 50mM acetate buffer at pH 4, was used for activity determination. The amount of librated glucose was measured by spectrophotometer at wave length 540 nm, using glucose kit (Biosystem, Spain, 2010).
2.7. Thermal Enzyme Stability
- The heat stability of enzymes has considerable importance from the application viewpoint; as best effectiveness can be attained only when enzyme finds its favorite conditions. The thermostability of invertases from S.pombe H and S.pombe L was studied by heating the invertase of S.pombe H at 80℃ and the invertase S.pombe L at 60℃ for 35 minutes. Then the residual activity of the enzymes was determined as described in 3.3.2 using glucose kit (Biosystem, Spain, 2010). Results were recorded as % relative activity.
2.8. Effect of pH on Enzyme Activity
- Variation of pH can have a significant effect on enzyme stability with most enzymes showing a strong dependence of activity on pH. The pH-dependant activity profiles of both enzymes were determined at room temperature with a 50 g/L sucrose solution in 50 mM buffer.The effect of pH on the enzymes S.pombe H and S.pombe L activities were determined by assaying the enzyme as mentioned before with the different buffered (50mM) sucrose mixture at pH’s 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0 and 9.0.
2.9. Effect of Substrate Concentration
- The effect of sucrose concentration on the enzyme reaction rates was studied by monitoring the enzyme activity in the reaction mixtures containing variable amounts of sucrose [0.3, 0.6, 1.2, 1.5, 1.8 M] at pH 4.5 at 37℃ and fixed concentration of the enzyme. Then the activity of the enzymes was determined using glucose kit (Biosystem, Spain, 2010).
3. Results and Discussion
3.1. Thermal Enzyme Activity
- The effect of temperature on the two invertase enzymes invertase from S.pombe H and of S. pombe L activity was determined by measuring the hydrolysis of sucrose at various temperatures ranging from 30 to 90℃. Among different temperatures, the maximum activity for invertase S.pombe H and S. pombe L was observed at 80℃ and 60℃, respectively (Fig.1). Probably it was due to the fact that reaction rate initially increased as temperature raised, due to kinetic energy of reacting molecules. However, as the temperature increased the kinetic energy of enzyme exceeded the energy barrier. It resulted in the breaking of weak hydrogen bonding and hydrophobic bonds that maintain the structure of enzyme. The inactivation of enzyme at low temperature and thermal denaturation at high temperature might cause decrease in activity [17]. High temperature is advantageous for industrial processes when high temperature is required.
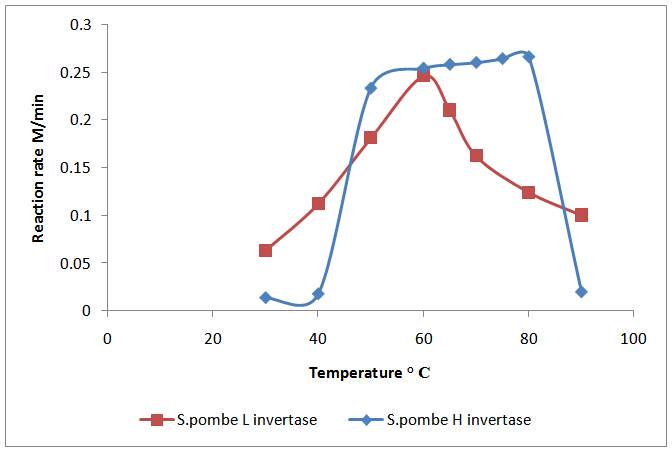 | Figure 1. Thermal enzyme activity (S.pombe H and S.pombe L invertase) |
3.2. Thermal Enzyme Stability
- The relationship of invertase S.pombe H and invertase S.pombe L activity with the change in heating time was shown in Fig. (2). It is obvious that there was an inverse correlation between increasing in the time period of heating and the enzyme stability. The results revealed that invertase S.pombe H had retained activity (about 15% of the activity) even after 35 min of incubation at 80℃. On the other hand, invertase S.pombe L was the most stable and had retained activity about 35% of the activity after 35 minutes of incubation at 60℃.
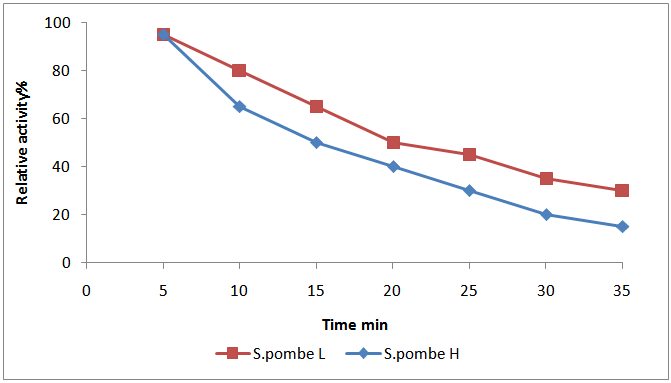 | Figure 2. Thermal enzyme stability of (S.pombe H and S.pombe L invertase) |
3.3. Effect of pH on Enzyme Activity
- The effect of various pH values on the invertase from S. pombe H and S.pombe L activity are depicted in Fig. 3. The graphic illustration elaborates that when the enzyme activity was calculated at different pH values, the invertase enzyme from S.pombe H was active in pH range 3.5 to 6 and the highest activity was found at 4.5 pH, while the invertase enzyme from S.pombe L was active in pH range 3 to 6 and the highest activity was found at pH 4.5. At pH above and below optimal level a decline in activity was possibly due to instability of protein structure. To explain the effect of pH on enzymatic activity, since enzymes are proteins, pH changes will profoundly affect the ionic character of amino and carboxylic acid groups on the protein and will therefore markedly affect the catalytic site and conformation of an enzyme. In addition the purely ionic effects, low or high pH values can cause considerable denaturation and hence inactivation of the enzyme protein. Moreover, since many substrates are ionic in character the active site of an enzyme may require particular ionic species for optimum activity. These effects are probably the main determinants of a typical enzyme activity-pH relation [22]. Kang et al. [23] stated that enzymes possess multimeric structures at different pH values and the ability of amino acids at the active sites to interact with the substrates depends on their electrostatic state. The decrease in activity at certain pH range shows that the enzyme faces some limitations. If the pH is not appropriate, the charge on one or all of the required amino acids at the active site is such that the substrate can neither bind nor react properly [24]. The result was in close agreement with those for Piciha anomala invertase (4.5) reported by Rodrigues et al., [25], Rhodotdrula glutinis invertase (4.5) reported by Rubio et al. [26], Leuosporidium antractium strain 171 invertase (4.5-4.75) reported by Turkiewicz et al. [27], Saccharomyces cerevisiae invertase (4.3) reported by Tanriseven and Dogan [28]. It was considerably lower than optimum pH 5.0 in Saccharomyces cerevisiae NRRLY- 12632 invertase [29], optimum pH 6.0 in A. flavus invertase 0) pH 6.5 in Escherichia coli invertase [30].
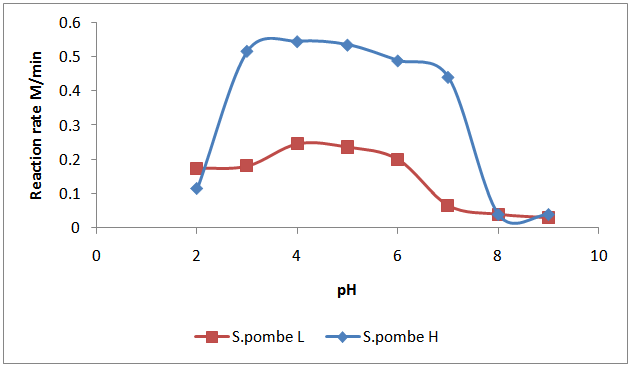 | Figure 3. Effect of pH on S.pombe H and S.pombe L invertase activity |
3.4. Effect of Substrate Concentration on Enzyme Activity
- Enzyme activity was very much dependent on sucrose concentration. Fig. (4a) and Fig. (4b) present the relation between initial rate and substrate concentration for invertase S.pombe H and S. pombe L, respectively. The enzyme activity increased with the increase in substrate concentration. Both enzymes activities increased with increasing sucrose concentration to reach a maximum activity at sucrose concentration of 1.9M, 1.2M for invertase S.pombe H and invertase S.pombe L, respectively, and thereafter the enzyme activity was reduced. At low concentration of substrate, the reaction rate is directly proportional to the substrate available. If the enzyme concentration is kept constant and the amount of substrate is increased, a point is reached in which increasing the substrate concentration does not increase the rate of the reaction. After this, the rate of enzyme reaction becomes steady and addition of the substrate will not have the positive effect [31].
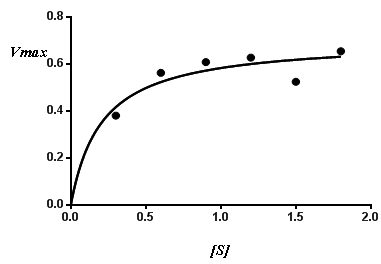 | Figure 4a. Substrate concentration-velocity date for the S.pombe H invertase enzyme (at constant enzyme concentration) |
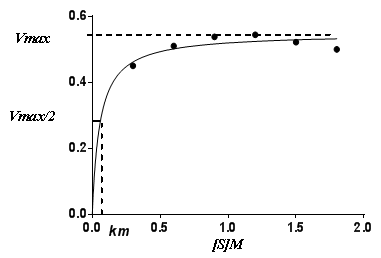 | Figure 4b. Substrate concentration-velocity date for the S.pombe L invertase enzyme (at constant enzyme concentration) |
4. Conclusions
- Invertase from S.pombe H exhibited maximum activity at pH 4.5 and temperature 80℃ and had retained 15% of activity after 35 minutes of heating 80℃, while, invertase from S.pombe L showed maximum activity at pH 4.5 and temperature 60℃; and retained more 35% of activity after 35 minutes of incubation at 60oC. It is highly recommended to produce invertase enzyme from yeast by utilization of food processing by-products fermented honey Duma xsas substrate could substantially lower the cost of production. The enzyme used in this study was partially purified so a further study could be done with more purified enzymes.
ACKNOWLEDGMENTS
- The authors would like to express their sincere gratitude to the Ministry of Higher Education and Scientific Research, Sudan for financing this study. Thanks are extended to all members and technicians of the Department of Food Science and Technology, Gezira University for technical assistance. Extend my thanks to Dr. Naglaa Gasmelsid in Department of Molecular Biology, University of Gezira.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML