-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2014; 4(2): 49-53
doi:10.5923/j.food.20140402.04
Effect of Germination on the Anti Nutritional and Toxic Factors of Cowpea
Malomo Olu., Alamu E. A., Oluwajoba S. O.
College of Food Sciences, Bells University of Technology, Ota, Nigeria
Correspondence to: Malomo Olu., College of Food Sciences, Bells University of Technology, Ota, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Germination had been speculated to possess the ability to eliminate if possible to some extent reduce some anti nutritional and toxic factors in legume, especially cowpea grains. The study was carried out on cowpea (vigna unguiculata), which was germinated for 5 days and the flatulence factor attributed to the oligosaccharides component of cowpea grains and the trypsin inhibitor associated with legumes were analysed. The result showed a drastic reduction of the trypsin inhibitor, measured by the trypsin inhibitor unit TUI/g from 1875 TUI/g in raw cowpea to 435 TUI/g on the fifth day of germination. The oligosaccharides component of raffinose family recorded as 6.5% for stachyose, 0.2% for raffinose and 1.9% for sucrose, on percent dry matter, while the second day the sucrose component increased astronomically to 11.4% with only traces of stachyose and raffinose. However, an unidentified carbon component of 3.5 % was observed. This trend continued to the fifth day, with 18.6% sucrose, traces of stachyose and 4.5% of the unidentified carbon compound. This unidentified carbon component was suggestive of a cyanogenic compound, built up within the grain as a defensive mechanism to ward off possible insect predators to the grain.
Keywords: Germination, Trypsin inhibitors, Oligosaccharides, Cowpea, Legumes
Cite this paper: Malomo Olu., Alamu E. A., Oluwajoba S. O., Effect of Germination on the Anti Nutritional and Toxic Factors of Cowpea, International Journal of Food Science and Nutrition Engineering, Vol. 4 No. 2, 2014, pp. 49-53. doi: 10.5923/j.food.20140402.04.
Article Outline
1. Introduction
- With the increase role that legume protein plays in the world food supply, the nutritive quality related to trypsin inhibitor level is of major concern. The presence of trypsin inhibitors has been demonstrated in a wide variety of cereals and legumes. [1] reported that an increase in nutritive value of soy flour paralleled the destruction of trypsin inhibitor activity. [2] suggested that trypsin inhibitors, along with other growth inhibitions that exists in addition to that resulting from phytomhaemaglutinins in soybean seeds, accounted for growth inhibition noticed when these seeds are fed to rats.Germination has been investigated as a means of reducing the anti-nutritional effects of proteases inhibitors. [3] found that trypsin inhibitor activity was not lost during germination of soybean. Similar results were also obtained by [4], for some germinated India pulses. However, [5] reported sprouts from navy beans. A similar trend was also reported from soybean sprouts by [6]. The loss soybean trypsin inhibitor activity was attributed to leaching during the daily washing of the sprouts [7]. [8] demonstrated only minor changes in soybean trypsin inhibitor activity using specific immunoelectrophoresis assay techniques. [9] concluded that the individual inhibitor components are in fluctuating. Although the trypsin inhibitor activity decline slowly after the 10th day of sprouting, it was still present in sufficient quantity to be of potential concern.A high trypsin inhibitor activity of horsegram and moth bean was reported by [10]. Although a high level of trypsin inhibitor was recorded for or horsegram (50 x 103 TIU/g seed) in the ungerminated form as compared to that of moth bean (4 x 103 TIU/g seed), the trypsin inhibitor activity in 72 hour germinated samples was reduced for by 15 percent in the case of horsegram and 40% in the case of moth bean. Cooking was found to inactivate the trypsin inhibitor activity to a considerable extent, although reduction was more pronounced in horsegram than in moth bean.[11] discovered that, a 9 hour sprouting of Phaseolus mungoroeus (a cross between Phaseolus mungo and Pheseolus aureus), the trypsin inhibitor activity decreased from 101.6 to 51.6 TIU/g seed .A similar trend was observed in parents P. mungo and P. aureus. They discovered that with the onset of germination, trypsin inhibitor activity increased slowly up to 295 TIU/g seed in 72 hours in the cross, and then declined to 46.6 TUI/g seed after a period of 9 days. P. mungo and P. aureus were also observed to behave in a similar manner. [12] reported the use of trypsin inhibitor to protect plants against insect predators.They attributed to slight increase in trypsin inhibitor activity at the onset of germination to the transformation of the dormant state of the seed to the vigorous metabolic state. This effect was found to be similar to that reported by [9]. [11] also found that the combination of germination and heating caused destruction of trypsin inhibitor. Trypsin inhibitor activity loss of about 71.8 percent was reported in P. mungoreous in a temperature range of 50-80℃ after 24 and 36 hour of germination. [13] found out that trypsin inhibitor in cowpea was affected by the reatment and fermentation with Rhizopus microsporous.
2. Changes in Flatulence Factors
- The ability of legume seeds to stimulate intestinal gas formation has been recognized for many years. Microbial fermentations in the large intestine are responsible for flatulence omponents such as hydrogen, methane and carbon dioxide [4]. However, oxygen and nitrogen may also present, which originate from swallowed air. In order that fermentation can occur, undigested or absorbed food residue must be available to the micro flora to act upon as this will serve as substrate. Many workers have attempted to identify factors in legume seeds which are responsible for the flatulence which occurs following their consumption. [15], using California small white bean, showed a 50 percent reduction in human flatulence volume after extraction of whole seed meal with aqueous alcohol. [16] showed similar change in flatulence reduction.Flatulence is widely accepted now to be due to ingestion of the indigestible oligosaccharides of the raffinose family [15]., [16] [17]; [18]. They are not digested as mammalian intestinal mucosa lacks a-galactosidase activity. However, bacteria in the lower intestinal tract are able to metabolise these sugars to carbon dioxide, hydrogen and methane, resulting in flatulence. Although flatulence is not considered a health problem, flatulence activity associated with legumes is a social discomfort to many people, and consequently, a disincentive to the increase use of legumes in the diet. During the germination of soybeans, raffinose disappeared by the end of 96 hours of germination and stachyose by the end of 144 hours [19]. [20]) found that, over a 13 day germination period, stychyose and raffinose declined rapidly in soybean cotyledons by day 3 and disappeared by day 9. The total soluble carbohydrate, predominantly sucrose, increase steadily during first five days and declined thereafter. A similar pattern of oligosaccharide change was noted for black eye and pink beans during a 4 day germination period by [21]. [11] also reported a decrease in raffinose and stachyose content with an increase in sucrose content. They found that the sucrose content doubled in 4 days of germination in Phaseolus mungoreous, phaseolus aureus and phaseolus mungo, while raffinose decreased for by about 90 percent and stachyose by about 93 percent. A similar study carried out by Reddy et al. (1980) [22] on black gram seeds showed that germination facilitated a disappearance of 86.8 percent of the oligosaccharides of the raffinose family. Results from studies carried out by [23] on mung beans and chick peas also showed a rapid decrease of the oligosaccharides in both legumes. This decrease was however slower in the case of chickpea.There are few data [24]; [25]; [26]; [27] dealing with the oligosaccharides of cowpea and the effect of germination on the oligosaccharides composition of cowpea Vigna unguiculata). The present study therefore aimed to provide additional information on the oligosaccharides composition of the cowpea dormant seeds and also to look at what changes these sugars undergo during germination.
3. Materials and Methods
3.1. Determination of Trypsin Inhibitor Activity of Cowpea Method Based on the Described by [5b]
- Thris-buffer (0.05M pH 8.2) containing 0.02M CaCl2: 6.05g tris-(hydroxymethylamino methane from sigma Chemical Co.) and 2094 CaCl2H20 dissolved in 500 ml of distilled water. The pH was adjusted to 8.2 and the volume made up to 1 litre with distilled water.Substrate SolutionForty milligrams of benzoyl-Dl-arginine-nitroanilide (BAPA) hydrochloride (Sigma Chemical Co) were dissolved in 1 ml of dimethyl sulphoxide (BDH) and diluted to 100 ml with tris buffer prewarmed to 37℃ while in use.Trypsin Solution 4 mg of accurately weighed trypsin (crystalline, salt free) (sigma chemical co) was dissolved in 200 ml .001M HCL. The solution was stored in the refrigerator.Cowpea SamplesRaw cowpea (ungerminated), 2,3,5 day germinated cowpea and processed cowpea were evaluated for their trypsin inhibitor activity .Preparation of cowpea samples for AssayOne gram of sample was extracted with 50 ml of 0.01M NaOH. The extraction time was 1 hour for the raw and germinated samples and 3 hours for the processed samples. The pH of the suspension was 9.5 – 9.3. The raw and germinated sample extracts were diluted 1;50 while the processed sample extracts to 1:10 with distilled water.ProcedurePortions (0, 0.6, 1.0, 1.4 and 1.8 ml of the diluted cowpea suspensions were pipette into duplicate sets of test tubes and adjusted to 2 ml with distilled water. After 2 ml of trypsin solution had been added to each test tube, the tube were placed in a water bath at 37℃. To each tube, 5ml of BAPA solution previously warmed to 37℃ were added, and exactly 30 minutes later reaction was terminated by adding 1 ml of 30 percent acetic acid. After thorough mixing, the content of each tube were filtered (Whatman No 541) and the absorbance of the filtrate was measured at 410nm against a reagent blank. The reagent blank was prepared by adding 1ml of 30 percent acetic acid to a test tube containing 2ml each of trypsin solution and 2ml distilled water followed by the addition of 5ml BAPA solution.Calculation of Trypsin Inhibitor Unit In calculating the result, one trypsin unit (TU) was arbitrarily defined as an increase of 0.01 absorbance units at 410 nm per 10ml of reaction mixture under the condition described here. Trypsin inhibitor was defined as the number of trypsin units inhibited (TUI).Determination of the components of oligosaccharides in Cowpea The oligosaccharide of raw, germinated and processed cowpea were determined according to the method described by Macrea & Zand Moghaddam (1978) [28].MaterialsMethanol water mixture (40:60 v/v); Carrez solution No 1 (10.6 g of potassium ferrocyanide (21.9 g of zinc acetate dehydrate and 2 ml glacial acetic acid made up to 100 ml with distilled water); acqueous standards were prepared individually for sucrose, raffinose and stachyose (10 mg ml -1 dry sugar).Sample PreparationDefatted finally ground sample (5g) was weighed into a 250ml round-bottomed flask and treated with 40 ml of the methanol-water mixture (40:60 v/v). The suspension was boiled under reflux in a water bath at 92℃ for 2 hours. After cooling suspension was transferred, and centrifuged at 20,000 g for 5 min. The residue was extracted twice more with 40 ml of boiling aqueous methanol and the final residue washed with 40 ml of distilled water.The combined extracts and washings were evaporated to dryness in vacuole below 50℃. The residue was dissolved in 15 ml of water and treated with 2 ml Carrez solutions No. 1 and 2 diluted to 20 ml with distilled water centrifuged the supernatant filtered and used directly for chromatography.AnalysisHigh pressure liquid chromatography was used.
4. Results
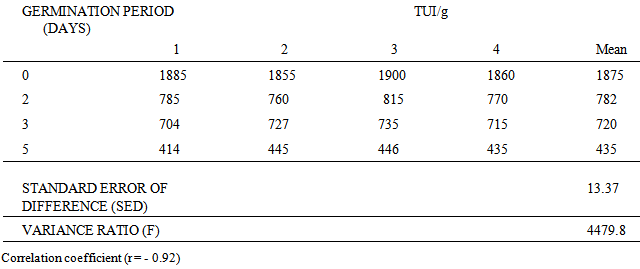
4.1. Effect of Sprouting on Trypsin Inhibitor of Cowpea
- The effect of sprouting on trypsin inhibitor activity of cowpea expressed as TUI/g was investigated. The results in Table 1 are based on 4 separate determinations. The results clearly showed that there was a reduction in the trypsin inhibitor activity of cowpea during sprouting. Reduction in trypsin inhibitor activity of cowpea and the period of germination have been found to be highly correlated (r = - 0.92).
|
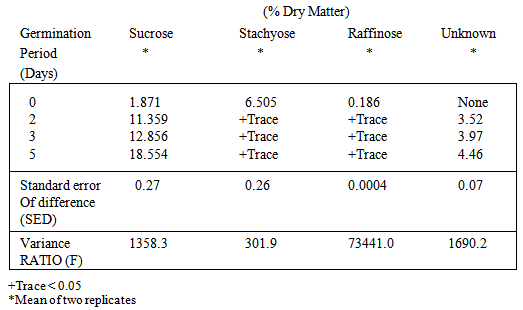
4.2. Effects of Sprouting on Oligosaccharides of Cowpea
- In raw cowpea, three sugar were identified; sucrose, stachyose and raffinose. Extracts chromate graphed in duplicate and mean peak area used for qualification. Aqueous standards were prepared individually for sucrose, raffinose and stachyose, containing 10 mg ml-1 dry sugar.The oligosaccharide composition obtained (Table 2) showed cowpea to be high in stachyose in the raw state (6.51%) with 1.87% of sucrose and small amount of raffinose (0.186%). There was a remarkable increase in the level of sucrose after the first day of sprouting this increase continued up to the fifth day. By the fourth and fifth day, sucrose content had increased by seven-and ten-fold, respectively. This changes were however, accompanied by the surprising disappearance of the sugars, right from the second day. In all the chromatograms obtained there was an unidentified peak, which proved to be neither pentose or hexose. The retention time is suggestive of a triose and was identical with that of deoxyglyceric aldehyde. The quantity of this unknown sugar increased with sprouting period.
|
5. Conclusions
5.1. Effect of Sprouting on Trypsin Inhibitor Activity of Cowpea
- The trypsin inhibitor activity of cowpea decreased with increasing germination period. A high correlation was found between reduction in trypsin inhibitor activity and the period of germination (r = 0.92). By the second day of sprouting, 58 percent of the original trypsin inhibitor activity present in raw unsprouted cowpea was removed. This fell to about 1/5th of the original trypsin inhibitor activity by the end of the fifth day.The values of the trypsin inhibitor shown in Table 1 are relatively small compared to what has been reported present in other legumes. Soy bean for instance, was reported by Collins and Saunders [7] to have a trypsin inhibitor activity as high as 226000 TUI/g. Subbulakshmi et al. [10] reported reduction in trypsin inhibitor activity of horsegram and moth bean during germination. A reduction of about 6 percent was reported for horsegram, and 40 percent in the case of moth bean. Similar report of reduction was also reported for soy bean during germination by Collins and Saunders [7].Gupta and Wagle [11] in their germination studies with Phaseolus mungoreous reported a slight increase in trypsin inhibitor activity with the onset of germination, which later rapidly declined with increasing germination period. The decrease in trypsin inhibitor activity during sprouting might be due to leaching during early stages of sprouting.
5.2. Effect of Germination on Oligosaccharides of Cowpea
- The oligosaccharides composition of cowpea in the raw state was of the order of 6.9% stachyose, 2.1% sucrose and 18.5% raffinose (Table 2). Progressive increase of sucrose occurred after the first day. Accompanying these changes, however, was a sudden disappearance of other sugars, right from the second day, and with an emergence of an unknown sugar, which was suggestive of a triose. The unknown sugar has an identical retention time on the chromatogram as deoxy glyceric aldehyde.Although similar trends were reported by [11], in their germination studies with Phaseolus mungoreus a total disappearance of the oligosaccharides and emergence of an unknown triose has not been previously reported during germination of legumes from this investigation, the significant amounts of the unknown triose produced during germination of cowpea make it of considerable interest and it needs to be investigated further. However, of immediate interest is the finding that oligosaccharides implicated as flatulence factors are completely removed by two-day germination of cowpea.According to the review by [29], sugars and transient starch are formed during the growth and maturation of plants as a result of photosynthesis. Carbohydrates is translocated, mainly in the form of sucrose, from the chloroplast via the phloem to the growing storage cells. Sucrose biosynthesis was postulated to be formed fromuridine-diphosphate-glucose (UDPG) and fructose-6-P as follows:UDPG + Fructose-6-P
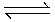 UDP + Sucrose – PSucrose – P
UDP + Sucrose – PSucrose – P  Sucrose + PThe sucrose is eventually reconverted into starch by reacting first with uridine-diphosphate (UDP) through the enzyme UDPG-fructose transglycosylase to form UDPG and fructose.Since fructose was not reported as seen during cowpea germination, it might have been further broken down by entering glycolytic pathway through fructose -1, 6-di-phosphate formed from fructose -1- phosphate. The fructose _1, 6-diphosephate is cleaved by the enzyme aldolase between the third and fourth carbon atoms to form two triose phosephate molecules. The increase in the unknown triose in cowpea during germination fits in with this metabolic pathway. The carbon group present might have reacted to form a cyanogenic group, forming a natural defensive compound by the plant rootlets to ward off insect predictors.
Sucrose + PThe sucrose is eventually reconverted into starch by reacting first with uridine-diphosphate (UDP) through the enzyme UDPG-fructose transglycosylase to form UDPG and fructose.Since fructose was not reported as seen during cowpea germination, it might have been further broken down by entering glycolytic pathway through fructose -1, 6-di-phosphate formed from fructose -1- phosphate. The fructose _1, 6-diphosephate is cleaved by the enzyme aldolase between the third and fourth carbon atoms to form two triose phosephate molecules. The increase in the unknown triose in cowpea during germination fits in with this metabolic pathway. The carbon group present might have reacted to form a cyanogenic group, forming a natural defensive compound by the plant rootlets to ward off insect predictors. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML