-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2014; 4(1): 20-26
doi:10.5923/j.food.20140401.04
Physicochemical Characterization of Non-alcoholic Beverages Produced from Malted Roasted Varieties of Maize (Zea mays)
P. T. Akonor, C. Tortoe, C. Oduro-Yeboah
Council for Scientific and Industrial Research - Food Research Institute, P. O. Box M20 Accra, Ghana
Correspondence to: C. Tortoe, Council for Scientific and Industrial Research - Food Research Institute, P. O. Box M20 Accra, Ghana.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Non-alcoholic malt beverages were produced with roasted malt from three maize varieties as Local, Obatanpa and Yellow corn. Chemical and physical properties of the beverages were determined by standard procedures for pH, titratable acidity (TTA), °Brix and colour. Acceptability test of the beverages was conducted using a trained laboratory-based panel of 20 members. The pH of beverage samples significantly differed (p < 0.05) among maize varieties and malting durations and ranged between 5.10 – 5.14 and 5.09 – 5.17 for the two treatments respectively. TTA was significantly different (p < 0.05) among the malting durations, with “96 hours malting” having the highest (0.19%). The TTA among the three varieties, however, was not significantly different. The °Brix increased as malting duration increased and colour were different (p < 0.05) among varieties and malting durations. Maillard and caramelization reactions, which occurred during roasting of the maize malts resulted in darkening of the non-alcoholic beverage and this was reflected in the low L* colour descriptor values. Sensory evaluation revealed significant differences between beverage samples malted over different periods. Chemical and visual properties of the non-alcoholic beverages from roasted maize-malt compared very well with those typical of other non-alcoholic malt beverage. Thus, roasting is a good unit operation to replace caramelization of sugar in the production of malt beverage from maize.
Keywords: Non-alcoholic beverage, pH, Roasted maize malt, Titratable acidity, °Brix, Colour, Acceptability test
Cite this paper: P. T. Akonor, C. Tortoe, C. Oduro-Yeboah, Physicochemical Characterization of Non-alcoholic Beverages Produced from Malted Roasted Varieties of Maize (Zea mays), International Journal of Food Science and Nutrition Engineering, Vol. 4 No. 1, 2014, pp. 20-26. doi: 10.5923/j.food.20140401.04.
Article Outline
1. Introduction
- Beverages are convenient and widely accepted for their thirst-quenching and refreshing properties as well as their ability to provide the needed energy for the body. They are produced from a wide variety of raw material of both plant and animal origin including fruits, vegetables as well as cereal grains, which provide proteins together with vitamins and minerals[1]. Beverages produced from cereal grains, both fermented alcoholic and non-alcoholic variants are consumed globally[2]. Cereals for use in beverage production are usually sprouted and dried in the process known as malting[2]. This modifies the grains physically, chemically and biologically[3. 4]. Desirable changes such as the hydrolysis of starch and protein into sugars and amino acids, respectively that occur in malted cereals used for the production of beverages and other cereal-based food have been widely studied[3-10]. Although the application of malted maize as a major source of hydrolytic enzymes for commercial brewing is not widely considered, its use in traditional non-alcoholic beverage production in Ghana is quite familiar. Customary non-alcoholic cereal beverage such as ŋmadaa (also Ahei, Asana or Liha) is processed from malted maize[11]. At the final stages of the production of these drinks, caramel is added to enhance flavour and give the dark brown colour characteristic of most malt beverages[11]. Roasting exposes food to dry heat for short periods and is known to impart desirable attributes. These pleasant qualities include texture[3], colour and flavour through the development of Maillard reaction products[12]. Other reported modification in roasted products include changes in amino acid profile in coffee[13], vitamins in barley malt[7], pasting, texture and thermal properties in barley[14]. Roasting of malt had been applied in brewing for colour and aroma development in beer[15]. A similar application to malted maize would subsequently improve the colour and enhance the flavour, aroma and other sensory characteristics of its non-alcoholic beverage. Adopting roasting would also eliminate the health risks associated with caramel usage and reduce the cost involved in producing the beverage. The objective of this study was to produce a non-alcoholic beverage from roasted maize malt without the addition of caramel, determine its physicochemical properties and assess its acceptability using a laboratory-based panel.
2. Materials and Methods
2.1. Raw Materials and Initial Treatment
- Three varieties of maize as Local, Obaatanpa and Yellow corn were procured from the Madina market in Accra, Ghana for the study. These varieties were selected based on availability, affordability and their application in the production of ŋmadaa: a traditional maize malt beverage. The grains were sorted and winnowed of all foreign materials; grain stalks debris and remaining cob parts. The different maize varieties (4.0 kg of each) were steeped at room temperature (28°C) in tap water (1:2 w/v). The setup was stirred once after 12 hours of steeping. After 24 hr of steeping, the steep water was drained off together with remaining chaff and other floating foreign material. After steeping, the weights of the maize varieties were 6.1, 5.8, 5.8 kg for Local, Obaatanpa and Yellow corn, respectively. Four units, each weighing 1.4 kg, from each variety of maize were germinated for 24, 48, 72 and 96 hr.
2.2. Malting and Roasting
- Sprouting of the grains was done at room temperature (28°C). The units (1.4 kg/unit) from the three varieties of maize were malted on mica surface covered with moist towels. The towels were remoistened with 200 ml of water sprinkled twice daily at regular intervals to keep the grains moist and active and prevent mould growth. After germinating for stipulated time (24, 48, 72 and 96 hr) each unit of experimental setup was dried in a convective dryer (Apex B35E, London) at 50°C for 18 hr and the vegetative parts were removed by rubbing grains between the palms. The dried malted grains were then roasted in an electric oven at 225°C (General Electric, USA) for 10 min stirring it intermittently. The roasted grains were cooled, milled into grits (Cemotec, 1090 Sample Mill, Sweden) and packaged in high density polyethylene bags.
2.3. Mashing
- Roasted malted samples from each variety were mashed in cold water (24.9°C) at 1:4 w/v and warmed at 65°C for 5 min. The mash was made to stand for 10 min to allow for sedimentation before it was filtered by gravity using a cheese cloth.
2.4. Formulation and Bottling
- Using sucrose as sweetener, the Pearson-square method was used to adjust the °Brix of the final malt beverage to 10° for samples whose °Brix was lower than the desired °Brix of 10 – 12°. The formulated malt beverage was then pasteurized at 75°C for 10 min in batches, using stainless steel pans. Immediately after pasteurization, the beverages were bottled hot and the bottles laid on their side for rapid cooling.
2.5. Colour
- Colour of the samples was determined using a Minolta Chromameter (CR-310, Japan). The colour values were expressed as L* (whiteness/darkness), a* (redness/ greenness) and b* (yellowness/blueness). The variation of colours were characterised by the total colour difference (∆E), which was calculated from the Hunter L*, a*, b* values using equation 1[16].∆E = (∆L2+∆a2+∆b2)1/2.................Equation 1
2.6. pH, °Brix and Titratable Acidity
- The pH of malted roasted beverage was measured using a pH meter (Jenway 3330, England) and °Brix was measured using a hand-held refractometer (HANNA RB 32, Germany). The titratable acidity was determined using titrimetry[17]. Malted roasted beverage sample was titrated against NaOH (0.1N) using phenolphthalein as indicator to a faint pink endpoint and total acidity expressed as percent of malic acid equivalent.
2.7. Sensory Analysis
- Trained laboratory-based panel of 20 members with previous experience in sensory evaluation assessed beverages in an acceptance test[18]. Beverages were presented in identical containers labeled with a different 3-digit code, to panelists. They were asked to indicate their overall acceptability of samples in their preferred choice of order on a 7-point Hedonic scale that ranged from “Like very much” (7) to “Dislike very much” (1). Panelists were instructed to rinse their mouths with water before evaluating subsequent samples. A serene atmosphere of good lighting and ventilation, as well as quietness was provided for panelists to conduct the evaluation[19].
2.8. Statistical Analysis
- The experiment was set up in a 2-way factorial analysis using three varieties of maize and four malting durations. ANOVA of the results was done using Statgraphics Centurion 16.1.11 (StatPoint Technologies Inc, USA, 2010) to compare means. Least Significant Difference (LSD) test was used to examine multiple comparisons between means at 5% significance level. All determinations were done in triplicates and data reported as Means ± Standard Error.
3. Results and Discussion
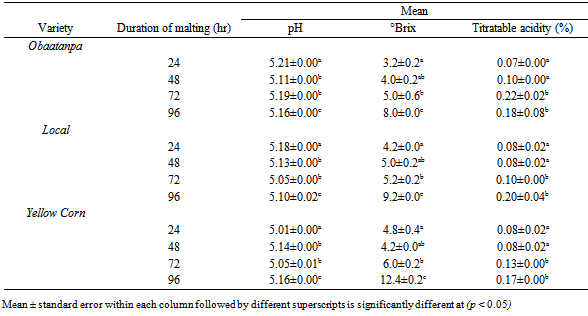
3.1. pH, °Brix and Titratable Acidity
- Non-alcoholic beverages from malted roasted Obatanpa variety generally had significantly higher (p < 0.01) pH, followed by Local and Yellow corn (Table 1). There was also a significant effect of the duration of malting on pH of the beverages (p < 0.01). Post hoc test (LSD) revealed that malting for 72 hr lowered the pH more than malting for 24, 48 and 96 hr. Interestingly, malting for 24 and 48 hr had the same effect on the pH of the beverages, while malting for 96 hr reduced the pH of the beverages the least. Furthermore, the interactive effect of malting time and maize variety had a significant effect on the pH of malt beverage samples (p < 0.01).
|
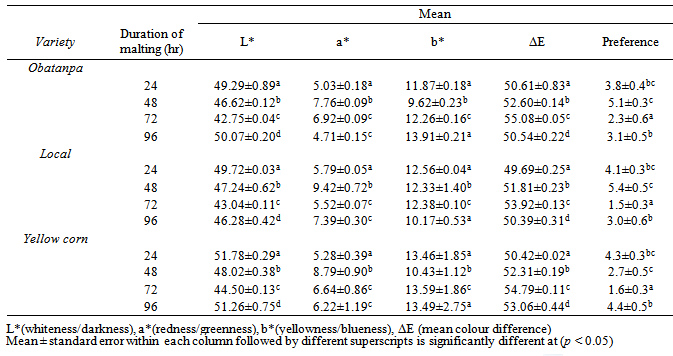
3.2. Colour
- Colour is an essential quality attribute that influences consumer judgment and preference of food. Colour indices (L*, a*, b* and ΔE) showed significant differences among varieties as well as the various malting periods, as shown in Table 2. The interaction term (malting duration and variety) of ANOVA for colour parameters also showed significant differences (p < 0.00). Variations in the colour of the beverages reflect the extent of colour development that occurred in their respective malts during roasting.
|
3.3. Sensory Evaluation
- Results of the acceptability test showed similarities between the three varieties of maize used as no significant differences existed between them (p < 0.72), an indication that any of the three varieties could be successfully applied in producing non-alcoholic malt beverage. However, on the Hedonic scale Local and Obatanpa had a mean score of 3.5 and 3.6, respectively suggesting that they were liked slightly, while Yellow corn was neither liked nor disliked as it retained a mean score of 3.3. This variety of maize has been shown to contain high levels of polyphenols and other compounds[33] and these may have imparted some bitterness and influenced some sensory attributes negatively hence beverages from this variety being the least acceptable by the 20-member panel.The test rather showed differences (p < 0.00) among samples malted to different extents. Malting beyond 48 hr did not contribute positively to acceptability of the beverages since those from 48 hr malting, averagely had higher acceptability rating than 24 hr malting, 96 hr malting and 72 hr malting notwithstanding the fact that 96 hr malting resulted in the highest Brix (Table 2; Figs 1, 2). This shows that the acceptability of the beverages is not based on its Brix alone but a combined effect of several attributes.
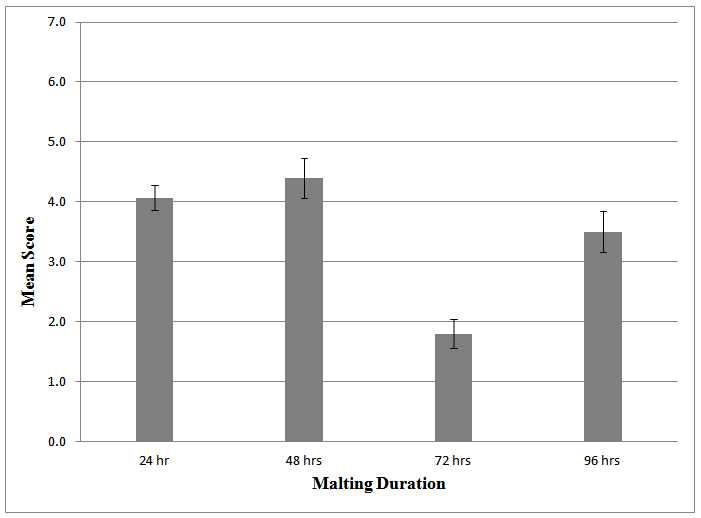 | Figure 1. Mean Score of beverage samples from different malting duration |
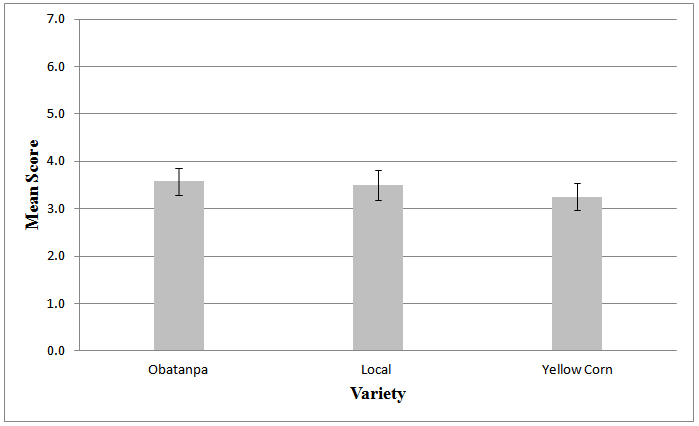 | Figure 2. ANOVA Score by variety of the maize |
4. Conclusions
- This study has shown that roasting can be applied to give malted beverages its characteristic color, rather than relying on commercial caramel. The beverages had the dark brown colour that characterizes malt beverages because of the Maillard and/or caramelization reactions that occurred as a result of roasting of the maize malt. The beverages pH, which were significantly (p < 0.05) influenced by the duration of malting ranged from 5.01 to 5.21. The °Brix and TTA of the beverages ranged between 3.2 – 12.4 and 0.07 – 0.22 %, respectively. These two indices were found to increase with an increase in malting duration. Colour parameters of the beverages also differed significantly (p < 0.05) according to variety and malting duration. Beverages from yellow corn were the least preferred among the three maize varieties and beverages from 48 hr malting were the most acceptable by the sensory panel.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML