-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2013; 3(3): 28-34
doi:10.5923/j.food.20130303.02
Investigation on Antioxidant and Antinutritional Properties of Sorghum Stem Sheath-ginger Extract Based Non-alcoholic Beverage
Adedeji T. O.1, Oluwalana I. B.2, Ade-Omowaye B. I. O.3
1Department of Food Science and Technology, Osun State Polytechnic, P.M.B. 301, Iree, Nigeria
2Department of Food Science and Technology, Federal University of Technology, P.M.B. 704, Akure, Nigeria
3Dept of Food Science and Engineering, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Nigeria
Correspondence to: Oluwalana I. B., Department of Food Science and Technology, Federal University of Technology, P.M.B. 704, Akure, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
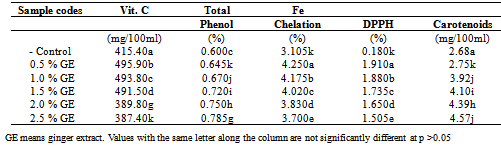
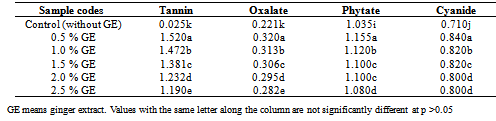
The anti-oxidant and anti-nutrients of laboratory produced non alcoholic beverage (obtained from aqeous extract of dried stem sheath of Sorghum bicolor with ginger extract was investigated. The samples were subjected first to pasteurization at 75oC for 15mins. The selected samples of non-alcoholic beverages were analysed for vitamin C, total phenol, Fe chelation, DPPH (1, 1-diphenyl -2-picryl hydrazyl, carotenoids, tannin, oxalate, phytate and cyanide. The beverage samples were found to contain substantial amount of total phenols ranging from 0.600-0.785%, vitamin C was present in adequate amounts (415.40-387.40) mg/100ml according to RDA requirements, Fe chelation ranged from 3.105-3.70%, DPPH) free radical ranged from 0.180-1.505%, while carotenoids content of the beverage samples ranged from 5.68-4.57mg/100ml. A decrease was recorded with total phenols, antioxidant activities which increase significantly at (p<0.5) with increasing concentration of the spices revealing the potentials of the spices, in enhancing the health promoting capacity of the beverage from the sorghum stem sheath. Moderate values were found for tannin (0.025-1.190) mg/100ml, oxalate (0.221-0.282) mg/100ml, phytate (1.035-1.050) mg/100ml and hydrogen cyanide (0.710-0.800) mg/100ml. The recorded anti-nutritional factors in all the beverage samples were within the safe levels.
Keywords: Anti-Oxidant, Anti-Nutrients, Samples, Ginger, Beverage
Cite this paper: Adedeji T. O., Oluwalana I. B., Ade-Omowaye B. I. O., Investigation on Antioxidant and Antinutritional Properties of Sorghum Stem Sheath-ginger Extract Based Non-alcoholic Beverage, International Journal of Food Science and Nutrition Engineering, Vol. 3 No. 3, 2013, pp. 28-34. doi: 10.5923/j.food.20130303.02.
Article Outline
1. Introduction
- Sorghum is an important staple food crop in African, South Asia and Central America, is the fifth major cereal crop in the world after wheat, rice, maize and barley[1]. It is also grown in the United States, Australia, and other developed nations for animal feed. Sorghum is most extensively cultivated in the drier Northern Guinea, Sudan Savannah and Greenland of African, Plains of India and United State of America[2]. It has grown in India and China for almost 2000 years. It is a vastly complex genus, embracing hundreds of variants of different characteristics and adapted to different ecological riches and with a variety of economic uses. It resembles maize in its vegetative character but different in having narrow leaves and a waxy bloom covering the leaves and stem. It also has a well-developed root/stem which is twice as efficient as that of maize although its leaf area is only half that of maize[3]. Historically, sorghum has been used in West Africa to color leather goods which include suitcases, shoes, baskets, hats and book covers. Traditionally, bundles of leaf sheaths are extracted in a laborious cottage –industry process. Other uses of sorghum include the production of industrial alcohol from the seeds, vegetable oil, broom-making, adhesives, waxes, sizing papers and cloth[4]. Sorghum bicolor are popularly used nationwide to prepare a gruel meal or pap. The gruel has been found to be highly nutritious[5]. There is a huge potential benefit, both economic and health wise if this food crop is developed as a natural food colorant in Nigeria. The stem has been used in the preparation of local medicine to treat anaemia and other related blood ailment. It has been reported to be used as an anti-malarial and anthelminthic[6]. The Sorghum bicolor L. Moench stem is sweet to taste and is found to contain some sugar and minerals; this sugary nature makes it to be easily chewed in African and Asia and is used for manufacture of syrup[7]. It is also used as color additive in cooking meals and taken as beverages when steeped or boiled in water in many homes in Nigeria[8]. The use of local spices to control the activities of micro-organisms in food has been reported[9];[10]. Apart from antimicrobial activities, spices are believed to have medicinal value (especially in African settings) and have desirable determinative influences on the overall organoleptic analysis when used. In view of these, it is therefore deemed fit to analyse the sorghum stem sheath beverage in combination with ginger for its nutritive value.
2. Materials and Methods
- Sorghum stem sheath in dry form and ginger were purchased from market in Oshogbo, Osun State. The method described by[11] was adopted with some modifications. The sorghum stem sheath was dry cleaned and further dried in an air oven at 30℃ for 6 hrs for moisture uniformity. The dried samples were milled separately into flour using a coffee mill and sieved through 450 μm aperture sieve. The flour samples were packed in air tight containers until utilized.The stem sheath flour / water ratio: 1/30 was used.The flour was soaked for 30 mins at ambient temperature before boiling for 30 mins. The extract was then filtered with clean muslin cloth to obtain clear filtrate. The filtrate was sweetened with food grade sucrose to a brix level of 10. The sweetened beverage was then dispensed into previously sterilized bottles before pasteurization at 75℃ for 15 mins. The beverage samples were then subjected to further investigations.
2.1. Spice Preparation
- The method described by[12] was adopted with some modification. Ginger stems were sorted and cleaned from extraneous materials and adhering particles. The cleaned stems were dried in an oven for 48hrs at 50oC to a moisture content of 10℃. The cleaned dried stems were pulverized/ milled into fine powdery form (0.5mm) using a hammer mill within few minutes of extraction to prevent loss of flavour. The milled spice was sieved packed in polyethylene film and stored at refrigeration (8 ± 2℃) until used. The flowchart for the process is described in Figure 1.
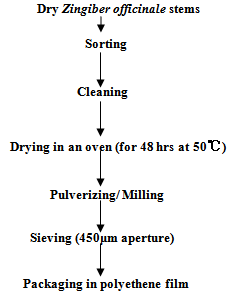 | Figure 1. Flow chart for spice preparation from dry Zingiber officinale |
2.2. Extraction of Crude Antioxidant Extract from Zingiber Officinale Powder
- Ten grams of finely ground spice was weighed into a thimble and extracted with 150ml of diethyl ether in soxhlet extractor for 2 hours. The crude extract was obtained by evaporation of the associated solvent and it was packaged in amber bottles and stored at refrigeration temperature (8 ± 2℃) until used. The percentage yield of the extract was calculated.
2.3. Beverage Formulation from the Stem Sheath Flour and the Spice Extract
- The filtrate obtained was mixed with the extracts of ginger (Zingiber officinale) at above concentrations. This implies a total of 6 samples including beverage without spice (control). The mixture was sweetened as described above before boiling for 30mins.The beverage samples were pasteurized as described above to obtain a ready to drink beverage. The flowchart for the process is described in Figure 2.
 | Figure 2. Flow chart for the production of spiced beverage samples from sorghum stem sheath flour with ginger |
2.4. Statistical Analysis
- All experiments were replicated twice and each analysis was duplicated indicating four determinations for each parameter. All generated data were subjected to statistical analysis using Statistical Analysis System[13] software package to determine significant differences (p<0.05) among the means.
2.5. Antioxidant Activity, Vitamin C and Carotenoid Evaluations
2.5.1. Determination of DPPH Radical Scavenging Ability
- The free radical scavenging ability of the extracts against DPPH (1, 1-diphenyl -2-picryl hydrazyl) free radical was evaluated as described by[14]. Appropriate dilution of the extracts (1 ml) was mixed with 1 ml, 0.4 Mm methanolic solution containing DPPH radicals, the mixtures was left in the dark for 30 mins and the absorbance was taken as 516 nm. The DPPH free radicals scavenging ability was subsequently calculated in percentage.
2.5.2. Determination of Total Phenol Content
- This was determined according to the method of[15]. Appropriate dilution of the aqueous extracts were oxidized with 2.5 ml of 10% Folin-Ciocalteau’s reagent (v/v) and neutralized by 2.0 ml of 7.5% sodium carbonate. The reaction mixture was incubated for 40 minutes at 45C and the absorbance was measured at 765 nm in the UV-Visible spectrophotometer (JENWAY 6405 Model, UK and England).
2.5.3. Fe2+ Chelation Assay
- This was determined using the method of[16]. Freshly prepared 500µM FeSO4 (150 µl) was added to a reaction mixture containing 168µl. 0.1 M Tris-HCl (pH7.4), 218 µl saline and the extracts (0-25% or 0-2.5%). The reaction mixture was incubated for 5 mins, before the addition of 13µl 0.25 % 1, 10-phenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer.
2.5.4. Determination of Vitamin C
- The determination of the vitamin C content of the sample was carried out using the technique of iodometric titration[17]. This involved adding 20 ml of 0.5 ml H2SO4 solution to 50 ml of the sample followed by the addition of the standard potassium iodide. Thereafter, 25 ml of the standard potassium trioxoiodate solution was pipetted into sample solution. The ascorbic acid in the sample was then analysed by generating excess water – iodine solution. The iodine was obtained from a standard solution of potassium trioxoiodate and potassium iodide. After the ascorbic acid has reacted, the remainder was titrated with a standard thiosulphate solution. The concentration of ascorbic acid in the sample was then expressed as mg ascorbic acid /100 ml of sample.
2.5.5. Determination of Carotenoids by HPLC
- Carotenoids was quantified by reversed –phase HPLC (brand, model, country)[18];[19]. 20 ml of ethanol was added to 2 ml of the sample already diluted with 1.8ml of water. After vortexing for 30secs, the samples was then extracted twice with n –hexane (1ml each time stabilized with 0.05% butylated hydroxyl toluene (BHT) and vortexed for 3 mins. The supernatant was removed, pooled and evaporated under nitrogen and reconstituted with 5 litres isopropanol and injected (4litres) into HPLC-system (Water, Eshborn, Germany). Accuracy and precision of the analyses was then verified using the control sample.
2.6. Anti-nutritional Factors Analysis
2.6.1. Oxalate Determination
- Oxalate content was determined using the method of[20]. About 5.0 g of the beverage sample was made alkaline with 10ml of 5% ammonium hydroxide. This was then made acidic with phenolphthalein (2 or 3 drops of this indicator) added, excess decolorized solution by drop wise addition of glacial acetic acid. Then 1.0cm3 (ml) of 5% calcium chloride was added and the mixture was allowed to stand for 3 hours after which were centrifuged at 3,000 rpm for 15 minutes. The supernatant was discarded and precipitated, washed thrice with hot water with thorough mixing and centrifuging each time. Then to each tube 2.0cm3 (ml) of NH2SO4 was added and the precipitate was dissolved by warming in a water bath (70℃ – 80℃). The content of each tube was titrated with freshly prepared 0.0IN potassium permanganate solution. Titration was carried on at room temperature (29℃) until the first pink colour appeared throughout the solution and was allowed to stand until the solution is colorless the solution was warmed to 70℃ – 80℃ and titration was continued until a pink colour persisted from at least 30 seconds.
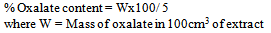
2.6.2. Phytate Determination
- Phytate was determined as phytic phosphate by the modified method of[21]. About 4g of finely ground samples were soaked in 100 cm3 (ml) of 2% HCL for 3 hours and then filtered. About 25 cm3 (ml) of the filtrate was placed in a 100 cm3 (ml) conical flask and 5 cm3 (ml) of 0.03% NH4SCN solution was then added as indicator. About 50cm3 of distilled water was added to give it the proper acidity. This was titrated with ferric chloride solution which contained about 0.005 mg of Fe per cm3 (ml) of FeCl3 used, the equivalent was obtained and from this, the phytate content in mg/100g was calculated.Iron equivalent=TV x 1.95Phytic acid =TV x 1.95 x 1.19 x 3.55
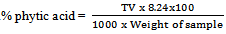 where TV =titration value
where TV =titration value2.6.3. Tannin Determination
- About 200 mg of finely ground sample was weighed into 50mls sample bottle and 10 ml of 70% aqueous acetone was added and properly covered. The bottles were put into an ice bath shaker and shake for 2 hours at 30C. Each solution was then centrifuged and the supernatant stored in ice. Also 0.2 ml of each solution was pipetted into test tubes and 0.8 ml of distilled water was added. Standard tannic acid solutions were prepared from a 0.5 mg/ml stock and the solution was made up to 1ml with distilled water. About 0.5% folin reagent was added to both samples and standard followed by 2.5 ml of 29% NaCO3.The solutions were then vortexed and allowed to incubate for 40 minutes at room temperature after which absorbance was read at 725 nm against a reagent blank concentration of the samples from a standard tannic acid curve[22].
2.6.4. Cyanide Determination
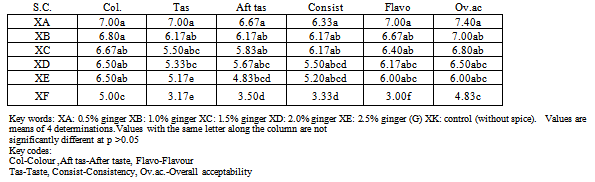
- About 5g of the sample was dissolved in 50mls of water in a conical flask, corked and was allowed to stay overnight. The solution was then filtered with media paper for cyanide determination. About 1 ml of the filtered solution was transferred into another conical flask and 4 ml of alkaline picrate solution was added and incubated at 50℃ in a water bath for 5 mins. Colour development and absorbance was taken at 190 nm. A blank preparation using 1ml distilled water was made. The cyanide content was extrapolated using standard curve and reported as mean of duplicate determination[5]. Sensory attributes of the beverage samplesThe sensory attributes of the formulated beverage samples are presented in Table 3. Beverage spiced with 0.5% ginger had the best ratings in all sensory attributes evaluated. Unspiced beverage (control) had the least ratings in all the sensory attributes indicating the potential of the spices inproducing acceptable beverage from sorghum stem sheath. Varying the concentration of ginger in the beverage did not result in significant (p<0.05) changes in almost all the studied sensory attributes.
3. Result and Discussion
3.1. The Antioxidant Properties, Total Carotenoids and Vitamin C of the Beverage Samples
- The anti-oxidant properties, total carotenoids and vitamin C content of sorghum stem sheath beverage are presented in Table 1. The total phenol of the beverage with ginger spice increased significantly at p<0.05 from (0.645 to 0.785)% with increasing concentration of ginger and the plain beverage was 0.60%. A significant increasing trend in the total phenol content with increasing concentration of ginger in the samples was observed from the information contained in Table 1. Addition of ginger resulted in 7 to 30% improvement in the total phenol contents of the beverage samples respectively. This observation confirms earlier report that spices have superior antioxidant capacity to berries, other fruits, vegetables and nuts[6].
|
|
|
3.2. The Anti-nutritional Factors of the Non-Alcoholic Beverage
- The anti-nutritional factor of the samples is presented in Table 2. Plain beverage had tannin content of 0.025mg/100ml while a range of 1.520-1.190mg/100ml was recorded for samples containing ginger extracts at varying concentrations respectively. A significant (p<0.05) decreasing trend in tannin content with increasing concentration of ginger in the samples was observed. All the spiced samples had significantly high tannin content than control sample. The relatively high tannin contents are from the spices used[34] reported that ginger contained 0.28mg/100g tannin. The tannin content of the non- alcoholic beverage which ranged between (1.520-1.190) mg/100ml was found to be much higher than that of sobo drink 0.035mg/100ml[35]. Plain beverage had oxalate content of 0.221mg/100ml while a range of 0.320-0.282mg/100ml was recorded for samples containing ginger extracts at varying concentrations respectively. A significant (p<0.05) decrease in oxalate content with increasing concentration of ginger in the samples was observed. The spice was observed to raise the level of oxalate in the beverage samples due to its own content or component. The oxalate content of sobo drink 0.615mg/100ml was much higher than that of the sorghum stem sheath beverage[36]. Plain beverage had a phytate content of 1.035mg/100g while a range of 1.155-1.080mg/100ml was recorded for samples containing ginger extracts at varying concentrations, respectively. A significant (p<0.05) decrease in phytate content with increasing concentration of the spice in the samples was observed. The addition of ginger into the beverage has contributed significantly to the elevation of phytate in the beverage samples[37]. However, the phytate content of the stem sheath beverage was much higher than that of sobo drink 0.032mg/100ml[34]. Plain beverage had cyanide content of 0.71mg/100ml while a range of 0.84-0.80mg/100ml was recorded for samples containing ginger extracts at varying concentrations respectively. A significant decrease in cyanide content with increasing concentration of ginger in the samples was observed. The minimal up surge in the cyanide value of the spiced beverages could be attributed to the additions from the spice. Cyanide content of sobo drink 0.016mg/100ml was found to be much lower than that of the stem sheath beverage[35];[34] Anti-nutrients have potentials in helping to reduce the risk of several deadly diseases in man if they are below the recommended or permitted levels in the body[38];[39];[40];[41];[42]. Reports shows that these chemicals reduce Low Density Lipoprotein (bad cholesterol) involved in depositing fat in the arteries[43], prevent blood clotting which can reduce the risk for a heart attack or a stroke. Sulphur compounds, which are examples of phytochemicals, are known also to reduce the cholesterol production in the body and through that keep the blood pressure down[43];[44]. They do this either by working alone or in the combination with vitamins and other nutrients in foods[45].
4. Conclusions
- The antioxidant activities of the stem sheath beverages were comparable and in some cases higher than in some fruits and vegetables. Moderate levels of tannin, oxalate, phytate and cyanide were recorded in the material. Addition of ginger in varying concentrations had significant (p<0.05) influence on the nutrient profile of the formulated non-alcoholic beverage samples.A decrease was recorded with total phenols, antioxidant activities which increase significantly with increasing concentration of the spices revealing the potentials of the spices, in enhancing the health promoting capacity of the beverage from the sorghum stem sheath. The recorded anti-nutritional factors in all the beverage samples were within the safe levels.
5. Recommendations
- The following recommendations could be made from this study:The formulated beverage could serve as a refreshing drink to people of all social classes thereby replacing the heavily chemical containing drinks which are detrimental to human health.Industrial production of this beverage should therefore be encouraged, which would not only alleviate the longing for fluid intake in warm tropical climate but would also provide a cheaper and more nutritive drink than the sugar laden fizzy drinks in the market.The phytochemical studies support its traditional uses which may prove to be useful for clinical evaluation and development of commercial drugs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML