-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2012; 2(5): 96-100
doi: 10.5923/j.food.20120205.05
Effects of Seasonality on the Proximate Composition and Fatty Acid Profile in Cow Milk
Simionato J. I. 1, Ana Paula Hiroki 2, Marly Sayuri Katsuda 2, Mayka Reghiany Pedrão 2, Lucia Felicidade Dias 2, Nilson Evelazio de Souza 2
1Departmentof Basic Studies and Instrumental, State University Southwest of Bahia, Praça Primavera, 40, Itapetinga, 45700- 000, Brazil
2Department of Food Technology, Technological University of Paraná, Rua dos Pioneiros, 3131, Londrina, 860356-370, Paraná, Brazil
Correspondence to: Nilson Evelazio de Souza , Department of Food Technology, Technological University of Paraná, Rua dos Pioneiros, 3131, Londrina, 860356-370, Paraná, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
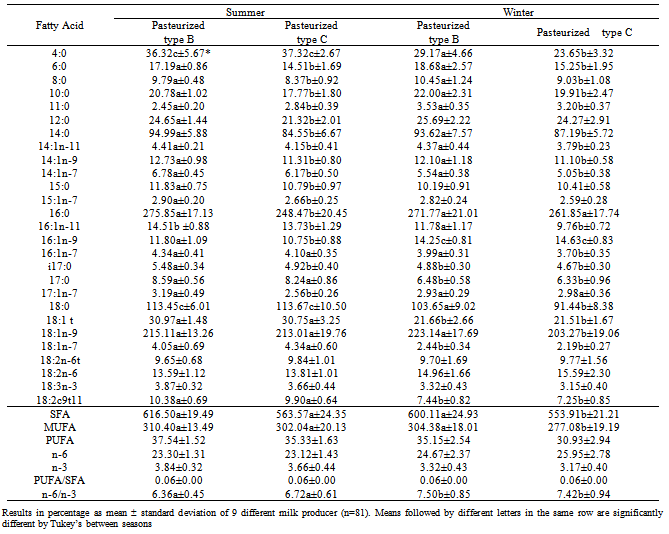
The present study investigated the influence of seasonality (summer and winter) on the proximate composition and on the amount of polyunsaturated fatty acids in milk fat. The amounts of lipid and protein were greater in the winter. The quantity of conjugated linoleic acid (CLA, rumenic acid, 18:2c9t11) in type B pasteurized milk (10.38 ± 0.69 in summer and 7.44 ± 0.82 in winter) was higher than in type C milk (9.90 ± 0.64 in summer and 7.25 ± 0.85 in winter) in both seasons. The amount of trans linolelaidic acid (18:2n-6t), linoleic acid (18:2n-6) and linolenic acid (18:3n-3) had no variation between seasons and types of milk. Furthermore, the n-6/n-3 ratio of the milks analyzed after pasteurization has reduced significantly from (p<0.05) 7.50 ± 0.85 and 7.46 ± 1.71 in winter to 6.36 ± 0.45 and 6.72 ± 0.61 in summer for milk Type C and B, respectively.
Keywords: Linoleic Conjugated Acid, Rumenic Acid, Cow Milk, Seasonality
Cite this paper: Simionato J. I. , Ana Paula Hiroki , Marly Sayuri Katsuda , Mayka Reghiany Pedrão , Lucia Felicidade Dias , Nilson Evelazio de Souza , "Effects of Seasonality on the Proximate Composition and Fatty Acid Profile in Cow Milk", International Journal of Food Science and Nutrition Engineering, Vol. 2 No. 5, 2012, pp. 96-100. doi: 10.5923/j.food.20120205.05.
Article Outline
1. Introduction
- Pasteurization is a thermal treatment applied to foods, including milk, to make them microbiologically safe for consumption. The most usual pasteurization process consists of raising temperature to 72℃ for 15-20 s[1]. Many researchers have studied how thermal processes affect the nutritional and organoleptic properties of foods. The pasteurization affects mainly protein structures[1-4]. However, few reports have demonstrated the effect of pasteurization on the composition and amounts of fatty acids[5]. Fat is one of the most abundant components of milk, and the most variable one. Its concentration and composition are more influenced by nutrition and environmental conditions than its other fractions[6, 7]. Conjugated linoleic acid (CLA) is a fatty acid present in ruminant milk fat. CLA isomers have different physiological effects. The biological activity of two of them, 18:2 t10c12 and 18:2 c9t11, have already been demonstrated. The first is a potent inhibitor of the fat synthesis in milk and responsible for the redistribution of fat in the muscle, being able to reduce fat mass and increase lean mass. The other has antitumoral properties, reducing the incidence of breast cancer[7-12]. CLAs may be produced synthetically and by the organism of ruminants in two ways: a) by incomplete biohydrogenation of dietary fatty acids and b) endogenously through the desaturation of trans-vaccenic fatty acid (18:1n-7t) by the enzyme stearoyl-CoA desaturase or ∆-9-dessaturase present in mammary glands and in adipose tissue. As 18:1n-7t is produced mainly by ruminal biohydrogenation, most CLA sources are ruminant products[13, 14]. Although the biological activities of c9t11 and t10c12 had already been confirmed, other CLA isomers and their interactions need further studies to determine their possible effects on human health. Furthermore, the use of CLA isomers in a variety of functional foods is a possibility that must not be ignored. However, it is also necessary to investigate the quantities of the isomers present in different food matrices. The separation and quantification of these isomers in dairy products by gas chromatography is challenging. Determining their mean amounts will enable setting a safe intake limit that is simultaneous suitable for the ingestion of CLA and saturated fatty acids. In this work, analyses on proximate composition and fatty acid quantification were performed focusing on conjugated linoleic acid (rumenic acid, 18:2c9t11), and omega-3 and omega-6 essential fatty acids in milks produced during two seasons (winter and summer) in Southern Brazil.
2. Materials and Methods
2.1. Sampling
- Raw milk from 430 dairy cows aged from 3 to 7 years in lactation period (150 days) was analyzed. The animals were from 9 farms in Middle East of Paraná State and had excellent health. The animals were given feed complemented with high-quality forage ad libitum. Forage consisted of predominantly mombaça (Panicum maximum cv. Mombaça), tanzania (Panicum maximum cv. tanzania), and brachiaria (Brachiaria brizantha cv. Marandu) grasses. The milk containers were immediately cooled to 4℃ after sampling and the milk was pooled, pasteurized (72℃ for 15-20 s) for milk type B (MB) and C (MC) and frozen. Sampling were conducted in three consecutive weeks in January and July 2009 (summer and winter, respectively). The weekly samples (n=81) were analyzed in triplicate.
2.2. Chemical Analysis
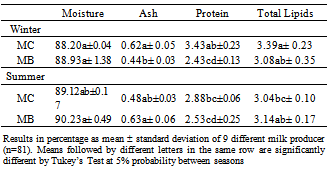
- Moisture was determined according to the AOAC method[15] by oven heating (105℃) and the ash content, by oven incineration (600℃). Nitrogen was determined by the Kjeldahl method.Total lipids were determined by the Folch[16] method with chloroform, methanol, and water in the ratio of 2:1:1.
2.3. Preparation of Fatty Acid Methyl Esters
- Fatty acid methyl esters were prepared as described by Bannon et al.[17] with modifications. To a screw cap tube with approximately 150 mg lipids was added 5.0 mL of 0.25 mol L1 sodium methoxide in methanol diethyl ether (1:1). The tube was stirred vigorously for about 3 min. The mixture was added with 3.0 mL isooctane and approximately 15 mL of a saturated sodium chloride solution. The tube was vigorously stirred again and left at rest for phase separation. The supernatant was collected in labeled Eppendorf tubes for later chromatographic analysis. The original method used short heating under reflux after the addition of the transesterification reagent; however, this was not done to prevent the isomerization of the conjugated linoleic acid dienes.
2.4. Chromatographic Analysis of Fatty Acid Methyl Esters
- Fatty acid esters were analyzed in gas chromatographer CP-3380 (Varian, EUA) equipped with a flame ionization detector and fused silica capillary column CP-7420 (100 m, 0.25 mm and 0.39 μm i.d., 100% bound cyanopropyl, Varian, EUA).The flows of gas (White Martins) were 1.4 mL min1 carrier gas (H2), 30 mL min1 auxiliary gas (N2), and 30 and 300 mL min1 for H2 and flame synthetic air, respectively. The sample split rate was 1/80. The injector and detector temperatures were both 240℃. The column temperature was set at 65℃ for 4 min, followed by a first 16℃/min ramp up to 185℃ for 12 min. The second ramp was set at 20℃/min up to 235℃, remaining at this temperature for 14 min. The total analysis time was 40 min. The peak areas were determined with the software Star (Varian). The injections were made in triplicate with injection volume of 2 μL. The fatty acids were identified based on the comparison of the retention times of the methyl esters with those of standards with geometric isomers c9t11 and t10c12 of linoleic acid (18919 and O-5626, Sigma, EUA).The limits of detection and quantification were estimated at 0.148 and 0.476 mg/g lipids, respectively, according to the ACS[18] considering the signal/noise ratio equal to 3 and 10, respectively, from successive dilutions of a standard solution of methyl arachidate.
2.5. Quantification
- Total lipid fatty acids were measured in mg g1 in relation to the internal standard, methyl tricosanoate (23:0), Sigma. Before transesterification, 1.00 mL of internal standard solution (1 mg mL1) was added to each sample and the solvent was evaporated under N2 flow.Fatty acid quantification was carried out after verifying the agreement between the theoretical and the experimental response factors.The sample fatty acid concentrations were calculated according to Joseph and Ackman[19] following the equation 1:C (mgg1)=Ax.M23:0.FRT / A23:0.MA. FCT (Equation 1)where: AX = Area of the fatty acid methyl estersA23:0 = Internal standard area M23:0 = Mass of Internal standard added to the sample (mg)MA = sample mass (g)FRT = theoretical response factor of fatty acid methyl esters,FCT = conversion factor to express results in mg fatty acids g1 total lipidsCertified reference material (power milk) was used to confirm the accuracy of the method. The reference material (RM-8435) was obtained from the Canadian National Institute of Standards and Technology (NIST). The accuracy of the method was confirmed by the analysis of the certified reference material (powder milk) with a quantitative recovery (>80%) of the analyzed fatty acids. This method may be applied to the quantification of fatty acids in commercial samples[20].
2.6. Statistical Analysis
- The results obtained were submitted to an analysis of variance (ANOVA) at 5% probability and the means were compared by Tukey’s test, using the software Statistica 7.0 [21].
3. Results and Discussion
- Table 1 lists the results of the analysis of variance for the chemical composition of milk after pasteurization in the summer and winter.
|
|
4. Conclusions
- In summary, the amount of CLA (rumenic acid) was higher in the summer, and the amount of trans linolelaidic acid (18:2n-6t), linoleic acid (18:2n-6) and linolenic acid (18:3n-3) had no variation between seasons neither between types of milk.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML