-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2012; 2(5): 76-84
doi: 10.5923/j.food.20120205.02
Enzymatic Liquefaction of Cell-Walls from Kent and Tommy Atkins Mango Fruits
Beatriz Brito 1, Fabrice Vaillant 2
1Instituto Nacional Autónomo de Investigaciones Agropecuarias (INIAP), Departamento de Nutrición y Calidad, A.P. 340, Quito, Ecuador
2Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), Tropical Fruits Department (FLHOR), B.P. 5085, 34032 Montpellier Cedex 1, France
Correspondence to: Fabrice Vaillant , Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), Tropical Fruits Department (FLHOR), B.P. 5085, 34032 Montpellier Cedex 1, France.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Table mango varieties discarded by the export market are generally not considered suitable for processing mainly because they yield too viscous fruit purées. The objective of this study was determining if appropriate enzymatic treatment can overcome this barrier. Cell-wall polysaccharides from mesocarp and pericarp of fully ripe table mangoes were characterized analyzing the alcohol-insoluble residues (AIR). Content of celluloses, hemicelluloses, lignin and soluble and insoluble pectin were assessed after selective extraction. After hydrolysis of main fractions, neutral sugars were determined by gas chromatography showing that xylose prevails (12-14 %) in the non-cellulosic fraction of insoluble cell walls from mesocarp, indicating predominance with cellulose, of xilan-type polysaccharides in mango flesh. Water insoluble AIR (WAIR) was incubated with commercial preparations, characterized for their main enzymatic activities and comprising balanced proportion of pectinases and cellulases with other different secondary activities. At equivalent 500 µl per kilogram of purée and at 45°C, solubilisation rates of uronids and neutral sugars reach respectively 100 and 90% only when xylanase activities were present. Then, a commercial enzyme preparation containing pectinases, cellulases and high xylanase activities was applied to native mango purée varying enzyme concentration and incubation time according to a central composite rotatable design. It was shown that percent of final suspended insoluble solids and Bostwick consistency could be considerably reduced in minutes, using a relatively low amount of enzyme preparation (150 l.L-1). Rheological properties of mango purées can be modulated easily according to incubation time and concentration of enzymatic solution to fit the needs of food industries.
Keywords: Mango, Cell-Wall Polysaccharides, Enzymes, Bostwick Consistency
Cite this paper: Beatriz Brito , Fabrice Vaillant , "Enzymatic Liquefaction of Cell-Walls from Kent and Tommy Atkins Mango Fruits", International Journal of Food Science and Nutrition Engineering, Vol. 2 No. 5, 2012, pp. 76-84. doi: 10.5923/j.food.20120205.02.
Article Outline
1. Introduction
- Global production of mango was forecasted to reachalmost 32 million tonnes by 2010, from which approximately 1,5 million were exported worldwide as fresh fruit[1]. Exported fresh fruits correspond to varieties that were selected for their firmness even at optimal maturity, as they must undergo considerable handling from field to international market, sometimes including hot-water treatments to reduce anthracnose incidence or eradicate fruit flies as required by the US market[2]. The varieties that can meet these technological requirements can be called table varieties, with Tommy Atkins and Kent among the main traded varieties.The high standards of quality imply that large quantities of fruit must be discarded by packing houses for being misshapen or having visual defects. Even in well managed plantations in Ecuador for instance, 20% to 30% of fruits are discarded in the fields and packing houses. These fruits are usually sold at low prices in local markets or even dumped in some cases. Although these mangoes have good internal properties, they are scarcely used by the juice industry because of poor technological properties such as low juice yield and highly viscous purée. Actually, other varieties are much more appropriate for juice production such as the Indian varieties Alphonso and Totapuri, which represent most of the international trade in mango purée[3, 4]. These varieties give off better aroma, have a higher juice yield and lower content of suspended insoluble solids than table mango varieties. Even so, the low value of the significant amount of discarded table mangos has become a major constraint to increasing the competitiveness of the export mango agro-industry. Special effort must therefore be made to process this fruit into a valuable product.Renewed interest in fruit products rich in dietary fibbers may also represent an opportunity for adding value to products prepared with table mangos[5, 6]. In fact, their technological characteristics, high viscosity and consistency, and low juice yield can be improved by using appropriate enzymes to degrade cell-wall polysaccharides, which are responsible for the purée’s rheological properties. Characterization of cell-wall polysaccharides has been studied by some authors for Alphonso[7, 8], Tommy Atkins and other varieties[8-11], but emphasis was put on studying ripening and natural tissue softening of mango fruit. Although studies on enzymatic liquefaction of viscous fruit juice to achieve better concentration rates are yet to be generally performed, all of them have been carried out on mango varieties appropriate for juice extraction and not on table mangoes[3, 12-15] except for Keitt variety[16]. Even these studies have been limited to testing commercial preparation, and little information is available on the relevant enzymatic activities that should be involved to reach the targeted technological effect. Consequently, for the two main table varieties (Tommy Atkins and Kent), specific studies are likewise necessary.This paper deals with (1) the characterization of cell-wall fruit polysaccharides of fully mature fruit, (2) the subsequent selection of an appropriate enzymatic preparation that can better liquefy cell-walls polysaccharides, and (3) application to purées to obtain different Bostwick consistencies and extend the industrial uses of discarded fruits. Actually, Bostwick consistency index is of very high practical interest for many industrial processes, in various fruit industries[17].
2. Materials and Methods
2.1. Raw Materials
- Green mangos were randomly selected from five packing houses. They were physiologically mature, but discarded by the export market for physical defects. All fruits were harvested from commercial plantations located in the Province of Guayas (Ecuador). Fruits were ripened at 22℃ and RH 75% for at least 8 days, and only coloured fruits (i.e. with more than 60% of the surface red for the variety Tommy Atkins and yellow for Kent) were selected.Fruits were manually peeled and mesocarp was separated and pulped with a processor, and then mash was sieved on a 2 mm diameter sieves to obtain native mango purée. Separated rind was also mashed under the same conditions.
2.2. Cell-Wall Materials
- Mashed mango or rind was suspended in five volumes of hot 95% (v/v) ethanol. The slurry was washed with 80% ethanol until the elute responded negatively to anthrone reagent. The raw alcohol-insoluble residue was freeze-dried, ground in a hammer mill and sieved (mesh size < 500 µm). It was then suspended in cold water (4℃) and washed thoroughly to remove soluble materials. The lost weight corresponded to the total content of water-soluble pectin (WSP). An aliquot of the recovered washing water was dialysed against distilled water, concentrated and freeze-dried to obtain part of the WSP for subsequent analysis.Starch and cytoplasmic proteins were partially removed, following the methodology presented by Brillouet, et. al.[18] Briefly, nonhydrosoluble residue was incubated with a protease (pronase) derived from Streptomyces griseus (and supplied by Boehringer-Mannheim, Germany), and then with a thermostable a-amylase (Thermamyl Liquid 60® from Novo Industrials, Denmark) and an amyloglucosidase from Aspergillus niger (Merck, Germany). The slurry was subsequently deproteinated and destarched, and centrifuged. It was washed thoroughly with distilled water to remove soluble materials and thus, obtain residue that was insoluble in both water and alcohol (WAIR). The WAIR corresponded to purified insoluble, cell-wall polysaccharides was freeze-dried, milled and sieved (mesh size < 500 µm). The AIR corresponded to the sum of total contents of WSP and WAIR.
2.3. Chemical and Physical Analysis
- Mango purée was analysed for pH, titratable acidity, dry matter, ash, soluble solids, total sugars and reducing sugars, using standard methods[19]. Vitamin C was assessed by the reflectometric method, using an ascorbic acid test (Merck’s Reflectoquant® kit).Cellulose and hemicellulose were extracted by selective fractionation of WAIR according to methods as described by Voragen et. al.[20]. Lignin was determined according to Effland.[21] Proteins were estimated, using the Kjeldahl method. Neutral sugars were analysed by gas chromatography (GC) (Shimadzu 14B, detector FID), featuring a Supelco SPB-225 column (30 m × 0.32 mm), after hydrolysing residues either by the Saeman (H2SO2 72%) or the trifluoroacetic acid (2 M) hydrolysis, and conversion of sugars to alditol acetates, according to the method adapted from Brillouet, et. al.[18] and Harris et. al.[22]. Only the higher content of neutral sugars obtained, whether by Saeman or TFA hydrolysis, were reported. Glucose liberated by TFA hydrolysis was reported as non-cellulosic glucose (NC glu), whereas cellulosic glucose was estimated as the difference between the TFA and Saeman hydrolyses.Total neutral sugars were measured, using the anthrone method[23] and deducing interference of galacturonic acid. Uronide material was determined by the m-hydroxy- diphenyl spectrophotometric method.[24] Starch content was determined following the enzymatic procedure described by Batey.[25] Methylation analysis of galacturonic acid was performed as described by Klavons et. al.[26] Suspended insoluble solids (SIS) were assessed as the percentage weight of the residue after centrifuging mango purée at 1000 g for 15 min. Consistency was assessed with a Bostwick consistometer, using 90 g of purée at a constant temperature (25±2℃), and measuring the distance (in cm) the pulp flowed under its own weight on a 15° slope for 30 seconds. Average of three consecutive tests was reported. Mesocarp firmness was assessed with a fruit pressure tester (Gullimex®), featuring a probe with a 0.8-mm diameter. Two measures per fruit were taken at opposite sides after removing peel.
2.4. Enzymatic Activity
- Activity of endo-1-4-ß-D-glucanase (Cx; EC 3.2.1.4), pectin lyase (PL; EC 4.2.2.10) Pectinesterase (PE; EC 3.1.1.11), Endo-polygalacturonase (PG; EC 3.2.1.15) and -L-arabinofuranosidase (EC 3.2.1.55) were assessed as described elsewhere[27]. The other enzymatic activities (1,4)-D-mannanase (EC 3.2.1.78), (1,4) -D galactanase (EC 3.2.1.89), and (1,4) -D-xylanase (EC 3.2.1.8) were assessed, using insoluble chromogenic substrates, that is, AZCL-galactomannan, AZCL-galactan and AZCL-xylan (all from Megazyme International Ireland), at 2% (w/v) in acetate buffer (25 mM) at pH4.5, 4.3, 4.7 respectively. Substrate solutions with the enzymatic preparation were incubated at 40 ºC for 10 min. Then, high molecular substrates were removed, employing 2.5 volumes of ethanol at 95% (v/v) and centrifuging at 1000 g. In the supernatant, the absorbance at 590 nm of the released dye-labelled polysaccharide fragments was measured. Enzymatic activity was determined by reference to a standard curve provided by the manufacturer (Megazyme International Ireland).
2.5. Enzymatic Treatment
- Two commercial enzyme solutions, Rapidase® Carrot Juice and Rapidase® Pomaliq 2F, were purchased from DSM (Seclin, France). Enzymatic hydrolysis of WAIR was performed as follows: 10 mg of WAIR was suspended in 10 ml of phosphate buffer at the pH of each fruit (pH 3.5 for Tommy Atkins and 4.7 for Kent). The enzyme concentrated between 5 and 50 µl per gram of WAIR, was added, and the whole incubated for 90 min between 30 and 60℃, and gently agitated. The enzyme was inactivated by heating for 5 min at 80℃, and the slurry centrifuged at 6000 g for 10 min. A blank without enzyme was also implemented. The supernatant was recovered for subsequent analysis of liberated sugars. All enzymatic preparations used were previously assessed for their endogenous content in AGU and in neutral sugars to report only sugars liberated from WAIR. Assays on native mango purées were done by introducing 1ml of diluted enzyme solution to 100 g of purée, incubating at 45℃ with gentle magnetic agitation. Then, purées were pasteurized at 80 ℃ during 5 min for subsequent analysis of SIS and Bostwick consistency.
2.6. Experimental Designs
- Two central composite rotatable designs (CCRD), , both with two variables (X1 and X2) corresponding to (1) temperature and enzyme concentration to assess response pattern of WAIR solubilisation; and (2) time and enzyme concentration to estimates responses on native mango purée. Responses (Y) were computed, using statistical software (JMP, v. 9.02, SAS Institute) to fit a second-order polynomial equation:Y = a0 + a1X1 + a2X2 + a11X12 + a22X22 + a12X1X2 (Eq 2)Statistical analyses, like coefficient of regression R2, analysis of variance and P-value associated were calculated. Variance of experimental error was assessed by repeating experiment at least three times in the centre of the experimental field. A P-value, called Plof was also used to test if the variance of residual corresponding to the lack of fit was similar to experimental error. Finally, a plot of residual versus predicted value was analysed to check for randomized pattern. All these analyses were provided by the software JMP (v. 9.02, SAS Institute). Once the model has been validated, response surfaces were presented as contour plot graph using the software SigmaPlot V7, 2001.
3. Results and Discussion
3.1. General Characterization of Mango Fruits
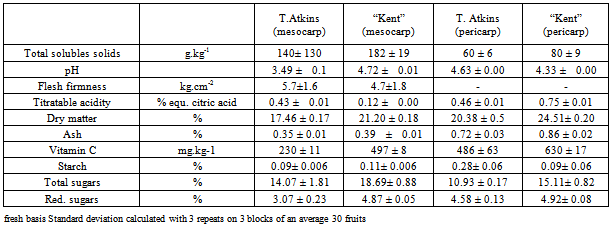
- At optimal maturity, both varieties Kent and Tommy Atkins differed slightly for several physico-chemical properties. Major differences were found for pH and acidity, firmness, and soluble solids and vitamin C contents (Table 1). Kent appears to be sweeter and less acid, and to contain 50% more vitamin C than does Tommy Atkins. The pericarp always presented higher vitamin C content than did mesocarp. The flesh is firmer in Tommy Atkins than for Kent.
|
3.2. Characterization of Cell-Wall Polysaccharides
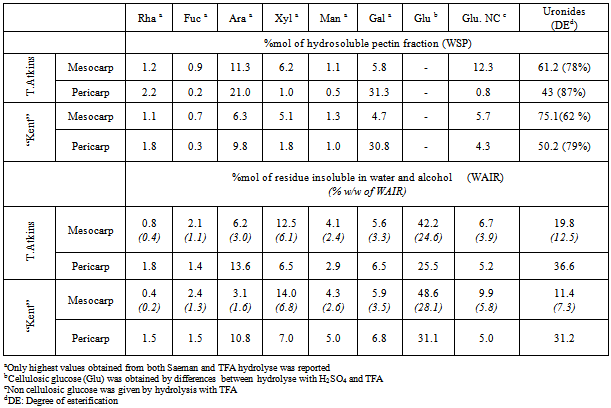
- The AIR, after deproteination and destarching, represented 1.7% and 1.45%, respectively, of the mesocarp from Tommy Atkins and Kent, and 7.2% and 4.1% of the pericarp (Table 2). The value for the mesocarp from Tommy Atkins is slightly higher than the 1.1 % found by other authors[10]. However, differences may be explained by the different extraction method followed. High content of AIR is responsible for the higher firmness of table mangos that are selected as better able to withstand mechanical stresses. The higher content of AIR in Tommy Atkins’s flesh explains the higher firmness observed previously. Both varieties yield almost the same amount of flesh (mesocarp). Although Kent has a thicker skin, it is counterbalanced by having a smaller seed that represents only 4% of the whole fruit, compared with 11 % for Tommy Atkins.Cellulose is the predominant component of AIR in the mesocarp, being around 37% for Tommy Atkins and 45% for Kent. Cellulose content is lower in the pericarp where soluble pectin is the major component (30% for Tommy Atkins and 40% for Kent). In the flesh (mesocarp), soluble pectin (WSP) represents only 32% of the AIR for Tommy Atkins and 25% for Kent. The content of hemicellulose is similar for both varieties. The amount of lignin in the Tommy Atkins mesocarp exceeded that in Kent, data, which can be also correlated with greater firmness.
|
|
3.3. Selecting an Enzymatic Preparation
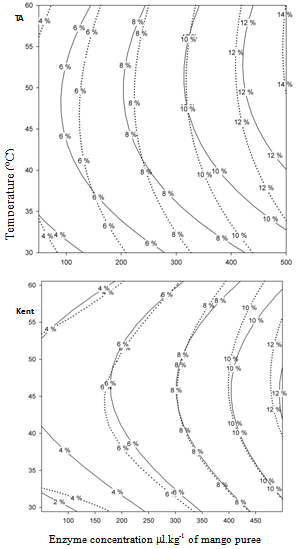
- The importance of cellulose and insoluble pectin in mango cell walls implies the need to use an enzymatic preparation able to hydrolyze at least both substrates. Nonetheless, the importance of other sugar polymers also may imply the use of other side enzymes, specifically, xylanases and arabinases. Seven commercial preparations were tested (data not shown), and among them, Rapidase® Carrot Juice and Rapidase® Pomaliq were able to solubilize the highest amount of insoluble cell walls from mango mesocarp (WAIR). The main spectrum of enzymatic activities of both commercial preparations has been published previously[18, 27]. They contain a high and balance amount of pectinases and cellulases, as well as other side activities, contrary to the other preparation tested (data not shown). The central composite rotatable design implemented, allowed testing different temperatures and enzyme concentrations, ranging respectively from 30 to 60ºC and from the equivalent of 50 to 500 µl per liter of mango purée (the mango purée being studied contains 10 ± 0.015 g of WAIR per kilogram of purée, as calculated from Table 2 for both varieties). The second-degree models obtained after computing the results of the experimental design gave rise to excellent statistical test values (r2 > 0.95; P≤0,01; Plof >10%). Corresponding response surfaces are presented as contour plot graphs in Fig. 1 and 2.Figure 1 shows, that for both preparations, at an equivalent of 500 µl per kilogram of purée, 100% of uronides (AGU) is released in the same way for both varieties as values around 12% w/w of WAIR are inclusively slightly higher than the ones obtained by chemical hydrolysis (Table 2). Figure 1 also shows that no significant differences between both preparations were found for the rate at which uronic material was released. The similar activity of the pectin lyase in both preparations may explain this result, as mango pectin is highly esterified. In fact, higher polygalacturonase and pectinesterase activities in Rapidase® Carrot Juice preparation[27] did not seem to improve uronides release. Figure 1 shows also that optimum temperature for pectinase activity is between 45 and 50℃.
3.4. Obtaining Mango Purées of Given Bostwick Consistencies
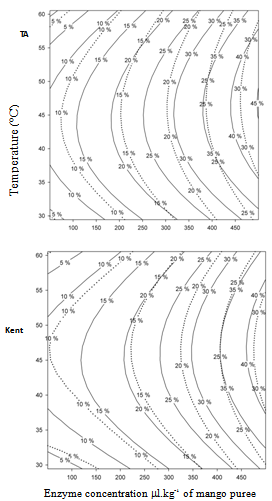
- Native mango purées obtained immediately after sieving are extremely rich in insoluble suspended solids (SIS; 84% for Tommy Atkins and 73% for Kent), giving rise to low Bostwick consistency (8 and 12 cm.30s-1 respectively for Tommy Atkins and Kent) (Table 5, run 1) only comparable to concentrated banana purée[17]. Purée from Kent variety appears to be less consistent than Tommy Atkins which is in agreement with previous results. As it can be observed in Fig. 3, enzymatic treatment with Rapidase® Pomaliq significantly decreases SIS and Bostwick consistency. By using relatively low concentrations of enzymes (150 ml per kilogram of purée) for 60 min of incubation, we could reduce SIS content by almost 30% and increase flow velocity on the Bostwick consistometer by approximately the same amount in both purées. For a very similar result on SIS, other authors[14] used much more enzyme solution (up to 2 ml kg–1), containing mainly pectinesterase and polygalacturonase activities.
|
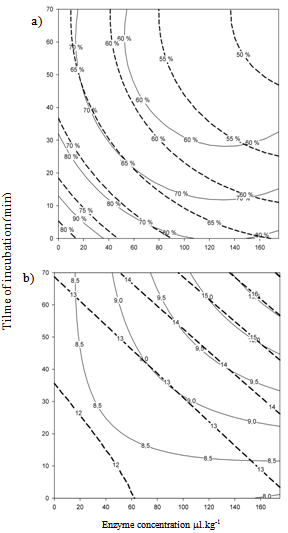 | Figure 3. Contour plot of responses on mango puree from “Tommy Atkins” (-----) and “Kent”(____) after incubation at 45 ºC with Rapidase Pomaliq®: a) % SIS; b) consistency (cm.min-1) |
4. Conclusions
- Mango table varieties are characterized by their firmness to endure mechanical stress during handling and transport of fresh fruits. Actually, their higher content of insoluble and soluble pectin, cellulose and xylan-type polysaccharides strengthens the structure of cell-walls, mainly in the flesh. As a consequence, purée resulting from fruits discarded by the export market was generally not considered suitable for the fruit juice industry. Nonetheless, we have shown that the appropriate choice of an enzymatic preparation can change this perspective. We have shown that the application of pectinases and cellulases is not enough and presence of xylanase, which is considered generally as a secondary activity in commercial preparation, was crucial to increase significantly the release of neutral sugars from isolated cell-walls from mango mesocarp. Xylanase may act on xylan-type polysaccharides but the effect on cell-wall solubilization is probably indirect, by facilitating access of pectinases and cellulases to their substrate. As a result, a commercial enzyme that presents high pectinase, cellulase and xylanase activities can reduce drastically content of suspended insoluble solids yielding in minutes a purée with rheological characteristics that can be modulated to fit better the needs of food industries. Additionally, the content of soluble and insoluble fibber of these purées could be high with subsequent health benefits for consumers. Therefore, although, purées of table mango varieties may be less flavorful than purées from other mango varieties, fresh table mangoes discarded from the export market only for visual defects, should be considered as highly valuable raw material for being used by food industries as a basis for different juice blends and smoothies with increasing market worldwide.
ACKNOWLEDGMENTS
- This study was carried out with financial support from PROMSA-MAG (Contract IQ-CV-077 with INIAP, Quito, Ecuador). The authors wish to thank S. Espin, M. Rodríguez, I. Samaniego, and M. Jaramillo for their valuable help and excellent technical assistance.
References
| [1] | FAO, Medium-term prospects for agricultural commodities. In FAO Corporate document repository, Department, E. a. S. D., Ed. 2010. |
| [2] | Yimyong, S.; Datsenka, T. U.; Handa, A. K.; Seraypheap, K., Hot Water Treatment Delays Ripening-associated Metabolic Shift in 'Okrong' Mango Fruit during Storage. J. Am. Soc. Hortic. Sci. 2011, 136 (6), 441-451. |
| [3] | Sreenath, H. K.; Sudarshana-krishna, K. R.; Santhanam, K., Enzymatic liquefaction of some varieties of mango pulp. Lebensmittel-Wissenschaft und Technologie 1995, 28, 196- 200. |
| [4] | Srivastava, J. S., Mango processing industries- a scenario. Indian Food Packer 1998, Nov-Dec, 43-51. |
| [5] | Gourge, C. M. P.; Champ, M. M. J.; Lozano, Y.; Delfort-Laval, J., Dietary fibre from mango by-products: characterization and hypoglicemic effects determined by in vitro methods. Journal of Agricultural and Food Chemistry 1992, 40, 1864-1868. |
| [6] | Vergara-Valencia, N.; Granados-Perez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Perez, L. A., Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. Lwt-Food Sci Technol 2007, 40 (4), 722-729. |
| [7] | Yashoda, H. M.; Prabha, T. N.; Tharanathan, R. N., Mango ripening-chemical and structural characterization of pectic and hemicellulosic polysaccharides. Carbohyd Res 2005, 340 (7), 1335-1342. |
| [8] | Yashoda, H. M.; Prabha, T. N.; Tharanathan, R. N., Mango ripening: changes in cell wall constituents in relation to textural softening. J Sci Food Agr 2006, 86 (5), 713-721. |
| [9] | Muda, P.; Seymour, G. B.; Errington, N.; Tucker, G. A., Compositional changes in cell wall polymers during mango fruit ripening. Carbohyd Polym 1995, 26 (4), 255-260. |
| [10] | Mitcham, E.; McDonald, R., Cell wall modification during ripening of "Keitt" and " Tommy Atkins" mango fruit. Journal of the American Society for Horticultural Science. 1992, 117 (6), 919-924. |
| [11] | Kratchanova; M; Benemou; C; Kratchanov; C, G., On the pectic substances of mango fruits. Elsevier: Kidlington, ROYAUME-UNI, 1991; Vol. 15. |
| [12] | Chauhan, S.; Tyagy, S.; Singh, D., Pectinolytic liquefaction of apricot, plum and mango pulps for juice extraction. International Journal of Food Properties 2001, 4 (1), 103-109. |
| [13] | Olle, D.; Baron, A.; Lozano, Y.; Brillouet, J., Enzymatic degradation of cell wall polysaccharides from mango (Mangifera indica L.) puree. Journal of Agricultural and Food Chemistry 2000, 48, 2713-2716. |
| [14] | Rastogi, N. K.; Rashimi, K. R., Optimisation of enzymatic liquefaction of mango pulp by response surface methodology. Eur Food Res Technol 1999, 209 (1), 57-62. |
| [15] | Yashoda, H. M.; Prabha, T. N.; Tharanathan, R. N., Mango ripening - Role of carbohydrases in tissue softening. Food Chem. 2007, 102 (3), 691-698. |
| [16] | Singh, N. I.; Dhuique-Mayer, C.; Lozano, Y., Physico-chemical changes during enzymatic liquefaction of mango pulp (cv. Keitt). J. Food Process Preserv. 2000, 24 (1), 73-85. |
| [17] | Perona, P., Bostwick degree and rherological Properties: An up-to-date viewpoint. Apllied rheology 2005, 15, 218-229. |
| [18] | Brillouet, J. M.; Rouau, X.; Hoebler, C.; Barry, J. L.; Carre, B.; Lorta, E., A new method for determination of insoluble cell walls and soluble nonstarchy polysaccharides from plant materials. Journal of Agricultural and Food Chemistry 1988, 36, 969-979. |
| [19] | AOAC, Fruits and fruit products. In Official Methods of Analysis of the Association of Official Analytical Chemists, Helrich, K., Ed. Association of Official Analytical Chemists: Arlington, USA, 2005; Vol. 2. |
| [20] | Voragen, A. G. J.; Timmers, J. P. J.; Linssen, J. P. H.; Schols, H. A.; Pilnik, W., Methods of analysis for cell wall polysaccharides of fruits and vegetables. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung 1983, 177, 251-256. |
| [21] | Effland, M. J., Modified procedure to determine acid-insoluble lignin in wood and pulp. Tappi 1977, 60, 143-144. |
| [22] | Harris, P. J.; Henry, R. J.; Blakeney, A. B.; Stone, B. A., An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohyd Res 1984, 127, 59-73. |
| [23] | Dubois, M.; Gilles, K. A.; Hamilton, J. K.; Robers, P. A.; Smith, F., Colorimetric method for sugars and related substances. Analytical Chemistry 1956, 28 (3), 350-356. |
| [24] | Blumenkrantz, N.; Asboe-Hansen, G., New method for quantitative determination of uronic acids. Analitical Biochemistry 1973, 54, 484-489. |
| [25] | Batey, I. L., Starch analysis using thermostable a-amylase. Starch 1982, 34, 125-128. |
| [26] | Klavons, J. A.; Bennet, R. D., Determination of methanol using alcohol oxydase and its application to methyl ester content of pectins. Journal of Agricultural and Food Chemistry 1986, 34, 597-599. |
| [27] | Brito, B.; Rodriguez, M.; Samaniego, I.; Jaramillo, M. I.; Vaillant, F., Characterising polysaccharides in cherimoya (Annona cherimola Mill.) puree and their enzymatic liquefaction. Eur Food Res Technol 2008, 226 (3), 355-361. |
| [28] | Olle, D.; Lozano, Y.; Brillouet, J., Isolation and characterization of soluble polysaccharides and insoluble cell wall material of the pulp of four mango (Mangifera indica L.). Journal of Agricultural and Food Chemistry 1996, 44, 2658-2662. |
| [29] | Prasanna, V.; Prabha, T. N.; Tharanathan, R. N., Pectic polysaccharides of mango (Mangifera indica L): structural studies. J Sci Food Agr 2004, 84 (13), 1731-1735. |
| [30] | Vaillant, F.; Millan, A.; Millan, P.; Dornier, M.; Decloux, M.; Reynes, M., Co-immobilized pectinlyase and endocellulase on chitin and nylon supports. Process Biochemistry 2000, 35, 989-996. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML