-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2012; 2(5): 70-75
doi: 10.5923/j.food.20120205.01
Application of Oregano Essential Oil Against Salmonella Enteritidis in Mayonnaise Salad
Janine Passos Lima da Silva 1, Bernadette Dora Gombosy de Melo 2
1Embrapa Food Technology, Av das Américas 29501, ZIP 23020-470, Rio de Janeiro, RJ, Brazil
2University of São Paulo, Faculdade de Ciências Farmacêuticas, Departamento de Alimentos e Nutrição Experimental, Laboratório de Microbiologia de Alimentos, Av Prof. Lineu Prestes 580 Bloco 14, 05508-900, São Paulo, SP, Brazil
Correspondence to: Janine Passos Lima da Silva , Embrapa Food Technology, Av das Américas 29501, ZIP 23020-470, Rio de Janeiro, RJ, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was applying oregano essential oil (OEO) from the Mediterranean regions, rich in Carvacrol and traces of p-Cymene and γ-Terpinene like prevention strategy against Salmonella Enteritidis (SE), controlling its multiplication in vegetable salad prepared with mayonnaise. An overnight culture at 30°C with 104CFU/mL of SE ATCC 13076 was inoculated in the salad containing mayonnaise and 0.2% (v/v) of OEO. A survival curve of SE in the salad under refrigeration (8℃) and at room temperature (30℃) after 0, 1, 2, 4, 8, 24 hours of incubation was done. A decrease in the multiplication rate of SE in the mayonnaise salad with OEO was observed. There was a reduction of > 0.5 log until 4 hours at 30℃ and until 24 hours at 8℃ in relation to the control. Our data suggest that OEO provides additional protection able to increase the safety of vegetable salad with mayonnaise contaminated with SE, but not for those subjected to temperature abuse. From Food Safety point of view this study has identified the use of OEO like natural antimicrobial able to reduce the SE growth, an enteropathogen associated with foodborne disease around the world in products derived from eggs such as mayonnaise.
Keywords: Oregano Essential Oil, Salmonella Enteritidis, Food Safety, Mayonnaise Salad
Cite this paper: Janine Passos Lima da Silva , Bernadette Dora Gombosy de Melo , "Application of Oregano Essential Oil Against Salmonella Enteritidis in Mayonnaise Salad", International Journal of Food Science and Nutrition Engineering, Vol. 2 No. 5, 2012, pp. 70-75. doi: 10.5923/j.food.20120205.01.
Article Outline
1. Introduction
- Foodborne Diseases (FBD) is caused by eating food that was contaminated by an infectious agent or a toxin produced by it [1]. According to the World Health Organization (WHO), 30% of people in industrialized countries suffer from FBD and in 2000 only at least two million people worldwide died of diarrhea caused or not by Salmonella[2];[3].Salmonellosis, a FBD caused by ingestion of Salmonella sp. occupies a prominent position in the field of public health throughout the world for its characteristics of morbidity and, in particular, the difficulty to be controlled. Salmonellosis is the third most commonly reported infectious disease in the United States, the first in Brazil (40.1% of bacterias) and the most frequently associated with human illness in the European Union [4],[5],[6]. Many foods can contain Salmonella and the most often related are raw meats, poultry, eggs, milk, dairy products and foods without heat treatment [7]. In the United States, salmonellosis is the cause of about 1.3 million FBD, with approximately 15,000 hospitalizations and 500 deaths per year, corresponding to 15.1% of the incidents followed by campylobacteriosis (13.8%) and shigellosis (6,4%)[8],[9],[10].Since the 1980's, these caused outbreaks are associated with the consumption of eggs, egg products and poultry products [11]. Salmonella Enteritidis (SE) is a relevant enteropathogen for the food industry, especially in foods that do not require heat treatment before consumption such as salads prepared with mayonnaise[12];[6],[10]. Mayonnaise is an egg-based product often involved in outbreaks of food poisoning, since it is consumed on a large scale because it is part of the preparation of salads, sauces and other dishes [13].The multiplication of microorganisms is determined by the mayonnaise pH, the type and concentration of acidulant, time and storage temperature. The aqueous phase with a pH below 4.1 ensures the microbiological safety, but the risk of contamination by Salmonella sp. only decreases with the use of pasteurized eggs. Other ingredients such as antimicrobial activity with salt, garlic, sugar and mustard mayonnaise also contribute to be considered a more safe product. However, the mayonnaise becomes a problem for public health from the moment it is mixed with other ingredients for preparing salads because the pH increases and, usually, these salads are packed in unappropriated temperatures [13].Several studies associated with control of SE in food have been developed in laboratory scale and some already applied at the level of industrial production to ensure food security. SE can be destroyed by heat treatment, but usually the salads with mayonnaise are not submitted to this type of treatment. This situation creates the need to use other methods for reduction or elimination of the pathogen within the concept of hurdle technology [14];[2];[15],[10].The multi-barrier technology can be defined as the application of conservation measures combined to increase the microbiological stability and sensory quality of foods, as well as their economic and nutritional properties. The barriers most commonly used in food preservation are temperature as a factor extrinsic and water activity (Aw), acidity (pH), redox potential, chemical and conservatives competing microorganisms like lactic acid bacteria (LAB) as intrinsic factors. In chilled foods is recommended to apply additional constraints to prevent the multiplication of microorganisms because the temperature of cooling is the biggest obstacle and in some cases, the only one. In case of temperature abuse occurrence in food distribution, that barrier is broken and there may be microbial multiplication in this food [16][17][15][18];[19]. Natural antimicrobials can be used as additional barrier as alternative procedure to control SE in these foods but their application is limited by the strong aroma that can negatively affect the sensory characteristics of the product. Govaris et al [20] analysed the antimicrobial effect of OEO against SE at 0.6 or 0.9% in minced sheep meat during storage at 4° or 10℃ for 12 days. At 4ºC, the population of SE in samples treated with OEO at 0.9% were kept below 1 log cfu/g [20]. Sensory evaluation showed that the addition of OEO at 0.6 or 0.9% in minced sheep meat was organoleptically acceptable [20]. However, the problem of unacceptable sensory characteristics can be reduced if used with other methods of preservation by hurdle technology. Whereas acidic nature of mayonnaise (pH 3.5) improves the ability of essential oils to penetrate the bacterial cell membrane and its application may be an additional protection to increase the safety of commercial or homemade mayonnaise salad, often subjected to temperatures higher than recommended [21]. This study aims to evaluate the antimicrobial activity of essential oil of oregano on Salmonella Enteritidis in mayonnaise salad refrigerated and subjected to temperature abuse.
2. Materials and Methods
2.1. Practical Proceedings to Evaluate the Antimicrobial Activity of Oregano Essential Oil
- Oregano essential oil (from Mediterranean zone) added on mayonnaise with 0.2% (v/v) final concentration [22]. Salmonella Enteritidis ATCC 13076 was maintained under refrigeration in Tryptic Soy Agar (TSA) (Oxoid). The inocula was resuspended in Brain Heart Infusion broth (BHI) (Oxoid) and incubated at 30℃ for 18 hours.To prepare the mayonnaise were used ingredients in the formulation of a standard commercial mayonnaise: soybean oil, sodium chloride (NaCl), vinegar 4%, Ethylenediamine tetraacetic acid (EDTA) and emulsifier, as Table 1.Four types of emulsifier were tested: egg powder, egg powder, pasteurized egg yolk liquid, which had a quality certificate indicating the absence of Salmonella sp. Mayonnaise was prepared in laminar flow, with sterilized equipment and solutions. The emulsifier (or egg yolk) was incorporated aseptically to the liquid phase consisting of vinegar, aqueous solutions of NaCl and EDTA at 4℃. After mixing these ingredients, soybean oil was slowly added to the aqueous phase under stirring in sufficient quantity (~ 70%) to form a stable emulsion. In this procedure was used a domestic "mixer" (Robot Classic - Mallory), previously cleaned with alcohol 70%.
2.2. Determination of Antimicrobial Activity
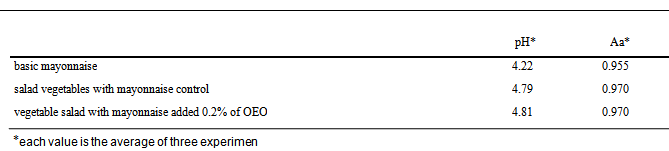
- To carry out the antimicrobial activity, 25 mL of culture in saline solution containing SE 104UFC/mL were inoculated with 225g Selection of Canned Vegetables (Carrefour SA) and mixed with 75g of mayonnaise containing 0.2% OEO. The procedure was repeated with mayonnaise without OEO, called “control sample”. Each of the mixtures was divided into twelve sterile bags in aliquots of 25g, six of which were stored at 8℃ and six to 30℃. At 0, 1, 2, 4, 8 and 24 hours, one of the bags was subjected to three decimal serial dilutions, which were plated on Xylose Lysine Deoxycholate agar (XLD) surface, in duplicate. After 24 hours incubation at 30℃, it did a count of colonies on the plates of XLD and the results were converted to log CFU/g of mayonnaise.The pH and Aw were measured in simple mayonnaise and mayonnaise mixed with salad vegetables. All experiments were analyzed three times, expressing the results as averages of three replications. For each batch of mayonnaise prepared, the pH and Aw were monitored.
3. Results
- The mayonnaise prepared with the four emulsifiers described in 2.3 item varied in emulsion stability as well as in pH during storage for 15 days under refrigeration. These data are shown in Table 2.
| |||||||||||||||||||||||
|
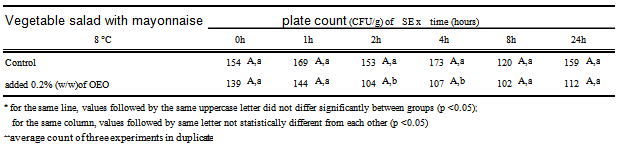
 | Figure 1. Survival of the SE vegetable salad with mayonnaise control and vegetable salad with mayonnaise containing 0.2% essential oil of oregano and the brand retained on 8℃. |
|
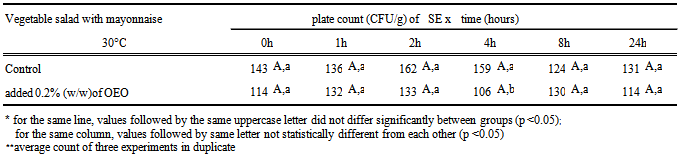
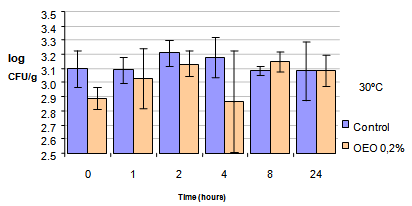 | Figure 2. Survival of the SE vegetable salad with mayonnaise control and vegetable salad with mayonnaise containing 0.2% oregano essential oil of the brand and maintained at 30℃ |
|
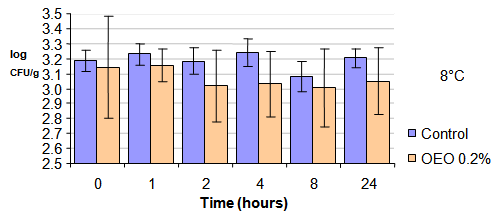
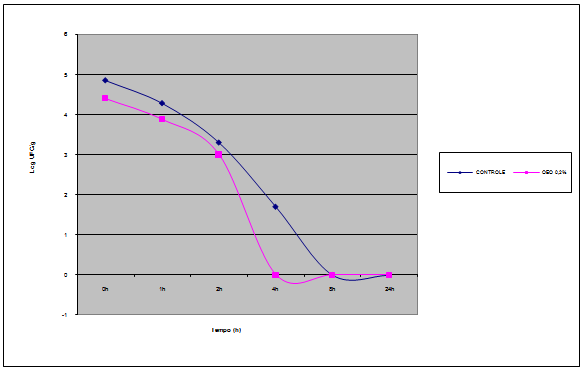 | Figure 3. Survival curve of Salmonella Enteritidis in mayonnaise with added 0.2% of OEO maintained to 8ºC |
4. Discussion
- There are few similar studies in scientific literature for a comparison of these results with those obtained in this study. Researchers[24] tested the survival of Salmonella Enteritidis inoculated into mayonnaise made with lemon juice (pH 4.4 to 4.5) supplemented with or without chitosan as antimicrobial and stored at 5 or 25℃ for 8 days. As a result, remained the same viability for 8 days at 5℃ in the absence of chitosan and decreased only 0.5 log CFU / g in the presence of chitosan.Numerous studies show that the in vitro effectiveness of antimicrobial agents is significantly reduced when tested in food model[24][25][26][27][28]. It is known that presence of lipids, carbohydrates, protein, salt has influence about antimicrobial activity. Researchers[29] tested essential oils of clove and cinnamon against SE in cheese with low (16%) or high (30%) lipid content of both oils tested were more efficient in cheese with low fat. The result found by these researchers may be due to the fact that fat to form a protective barrier around bacteria or the possibility of lipid fraction to absorb the essential oil. Thus, it one can speculated that the mayonnaise is not a suitable product for the application of an essential oil as antimicrobial agent, as is a product with approximately 70% fat hindered the action of the OEO is dispersed in fat globules while SE multiplies in the aqueous phase.Figure 3 show Survival curve of Salmonella Enteritidis in mayonnaise with added 0.2% of OEO maintained to 8℃ In mixed vegetable salad with mayonnaise handmade, the presence of 0.2% of OEO resulted in a reduction in the count of SE, thus becoming an effective barrier to inhibit the growth of the pathogen in the product.Increased security by increasing the concentration of OEO in these products is not always possible, they may become sensory unacceptable. It follows therefore that in reducing the risk of infection by SE in food mayonnaise or mayonnaise-containing foods must be added other barriers besides the temperature and the addition of OEO as a natural antimicrobial.As conclusion, it is important to observe that the use of biopreservatives (natural antimicrobials) is characterized as an additional barrier to the Good Manufacturing Practices (GMP) and HACCP program (Hazard Analysis Critical Control Point"), fundamental to Food Safety.
ACKNOWLEDGEMENTS
- We would like to thank the CNPq for financial support.
References
| [1] | BRASIL. MINISTÉRIO DA SAÚDE. AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Resolução nº 12, de 02 de janeiro de 2001. Aprova o Regulamento Técnico sobre Padrões Microbiológicos para Alimentos. Diário Oficial[da República Federativa do Brasil], Brasília, DF, 02 de janeiro de 2001. |
| [2] | BURT, S. (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. International Journal of Food Microbiology, 94, p.223– 253. |
| [3] | JONES, F.T. (2011) A review of practical Salmonella control measures in animal feed. J. Appl. Poult. Res. 20: 102-113. |
| [4] | AIKEN A M, C Lane , G K Adak Eurosurveillance, Volume 15, Issue 22, 03 June 2010 |
| [5] | CVE, 2011. Centro de Vigilância epidemiológica da Secretaria do Estado de São Paulo. Available at: |
| [6] | RAJASHEKARA, G., MUNIR, S., ALEXEYEV, M.F., HALVORSON, D. A., WELLS, C. L., NAGARAJA, K. V. (2000) Pathogenic role of SEF14, SEF17 and SEF21 fimbriae in Salmonella enterica serovar enteritidis infecti of chickens. Applied and Environmental Microbiology, Washington, v.66, n.4, p.1759-1763. |
| [7] | BAJPAI, V.K., BAEK, K-H, KANG, S.C. Control of Salmonella in foods by using essential oils: A review. Food Research International, Volume 45, Issue 2, March 2012, Pages 722-734 |
| [8] | ISAACS, S., ARAMINI, J., CIEBIN, B., FARRAR, J. A., AHMED, R., MIDDLETON, D., CHANDRAN, A. U., HARRIS, L. J., HOWES, M., CHAN, E., PICHETTE, A. S., CAMPBELL, K., GUPTA, A., LIOR, L. Y., PEARCE, M., CLARK, C., RODGERS, F., JAMIESON, F., BROPHY, I., ELLIS, A. (2005) An International Outbreak of Salmonellosis Associated with Raw Almonds Contaminated with a Rare Phage Type of Salmonella Enteritidis. Journal of Food Protection, v. 68, n.1, p.191–198. |
| [9] | SAMPATHKUMAR, B., KHACHATOURIANS, G.C., KORBER, D.R. (2003) High pH during trisodium phosphate treatment causes membrane damage and destruction of Salmonella enterica serovar Enteritidis. Applied and Environmental Microbiology, v.69, n.1, p.122-129. |
| [10] | SCHROEDER, C.M., NAUGLE, A.L., SCHLOSSER, W.D., ALLAN, T., HOGUE,T., FREDERICK, I., ANGULO, J., ROSE, J.S., EBEL, E.D., DISNEY, W.T., HOLT, K.G., GOLDMAN, D.R. (2005) Estimate of Illnesses from Salmonella Enteritidis in Eggs, United States, 2000 Emerging Infectious Diseases, 11, n.1. |
| [11] | INTERNATIONAL COMMISSION ON MICROBIOLOGICAL SPECIFICATIONS FOR FOODS. (2005) Microorganisms in Foods 6. Microbial Ecology of Food Commodities. 2nd ed. Kluwer Academic, New York. |
| [12] | BOONMAR, S., BANGTRAKULNONTH, A, PORNRUNANGWONG, S., TERAJIMA, J., WATANABE, H., KANEKO, K., OGAWA, M. (1998) Epidemiological analysis of Salmonella enteritidis isolates from humans and broiler chickens in Thailand by phages typing and pulsed-field gel electrophoresis. Journal of Clinical Microbiology, Washington, v.36, n.4, p. 971-974. |
| [13] | XIONG, R., XIE, G., EDMONDSON, A. S., MEULLENET, J-F. (2002) Neural network modeling of the fate of Salmonella enterica serovar Enteritidis PT4 in home-made mayonnaise prepared with citric acid. Food Control, 13, p.525-533. |
| [14] | BURT, S.A., REINDERS, R.D. (2003) Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Letters in Applied Microbiology v.36 n.3, p.162–167. |
| [15] | LEISTNER, L. (2000) Basic aspects of food preservation by hurdle technology: Review. International Journal of Food Microbiology, 55, p.181–186. |
| [16] | DE MARTINIS, E.C.P. e FRANCO, B.D.G.M. (1997) Inhibition of foodborne pathogens by bacteriocin-producing Leuconostoc sp. and Lactobacillus sake isolated from “lingüiça frescal”. Revista de Microbiologia, 28, p.284-287. |
| [17] | LEISTNER, L. (1999) Combined methods for food preservation. In: Shafiur Rahman, M. (Ed.), Handbook of Food Preservation. Marcel Dekker, New York, pp. 457– 485. |
| [18] | LIN, Y. T. , LABBE, R. G. , SHETTY, K. (2004) Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Applied and Environmental Microbiology, v.70, n.9, p.5672-5678. |
| [19] | ANGIENDA P. O., ONYANGO D.M., HILL D.J. (2010) Potential application of plant essential oils at sub-lethal concentrations under extrinsic conditions that enhance their antimicrobial effectiveness against pathogenic bacteria. African Journal of Microbiology Research, v. 4, n. 16, p. 1678-1684. |
| [20] | GOVARIS, A., SOLOMAKOS, N., PEXARA, A., CHATZOPOULOU, P. S. (2010) The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. International Journal of Food Microbiology, 137, p. 175–180. |
| [21] | KISKÓ, G., ROLLER, S. (2005) Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiology, 5:36. |
| [22] | SILVA, J.P.L., DUARTE-ALMEIDA, J.M., PEREZ, D.V., FRANCO, B.D.G.M. (2010) Oregano essential oil: influence of the chemical composition on the inhibitory activity against Salmonella Enteritidis. Ciência e Tecnologia de Alimentos, v. 30, supl 1, p. 136-141. |
| [23] | DAVIDSON, P.M., NAIDU, A.S. Phyto-Phenols. In: Naidu, A.S. (Ed.) (2000 Natural Food Antimicrobial Systems. CRC Press, Boca Raton, FL, pp. 265–294. |
| [24] | ROLLER, S., COVILL, N. (2000) The Antimicrobial Properties of Chitosan in Mayonnaise and Mayonnaise-Based Shrimp Salads. Journal of Food Protection, vol. 63, n. 2, p 202–209. |
| [25] | POL, I. E.; MASTWIJK, H.C.; SLUMP, R.A.; POPA, M.E.; SMID,E.J. (2001) Influence of food matrix on inactivation of Bacillus cereus by combinations of nisin, pulsed eletric field treatment and carvacrol. Journal of Food Protection, v. 64, n. 7, p. 1012-1028. |
| [26] | LIS-BALCHIN, M., BUCHBAUER, G., HIRTENLEHNER, T., RESCH, M. (1998) Antimicrobial activity of Pelargonium essential oils added to a quiche filing as a model food system. Letters in Applied Microbiology, v.27, p.207-210. |
| [27] | LEUSCHNER, R. G. K., ZAMPARINI, J. (2002) Effects of spices on growth and survival of Escherichia coli O157 and Samonella enterica serovar Enteritidis in broth model systems and mayonnaise. Food Control, 13, p.399-404. |
| [28] | RATTANACHAIKUNSOPON, P., PHUMKHACHORN, P. (2010) Antimicrobial Activity of Basil (Ocimum basilicum) Oil against Salmonella Enteritidis in Vitro and in Food Bioscience, Biotechnology and Biochemistry, v. 74, n. 6, p. 1200-1204. |
| [29] | SMITH-PALMER, A., STEWART, J., FYFE, L (2001) The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiology, v. 18, n. 4, p. 463–470. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML