-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
2012; 2(4): 54-62
doi: 10.5923/j.food.20120204.02
The Use of Cassava, Sweet Potato and Cocoyam, and Their By-Products by Non – Ruminants
D. F. Apata 1, T. O. Babalola 2
1Department of Animal Production, University of Ilorin, Nigeria
2Department of Animal Science Landmark University, Omu-Aran, Nigeria
Correspondence to: D. F. Apata , Department of Animal Production, University of Ilorin, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
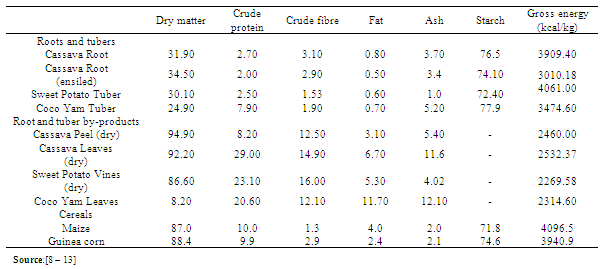
As the search for alternative sources of energy for non ruminants continues, this review was undertaken to examine the potential value of cassava (Manihot utilissima Pohl.), sweet potato (Ipomea batatas Poir.), cocoyam (Xanthosoma sagittifolium Schott.) and their by-products as dietary supplements for non ruminants. Studies on these roots and tubers showed that, on a dry-weight basis, contained 2.0 – 7.9% crude protein, 0.3 – 3.1% crude fibre and 72.4 – 77.9% starch. The practical use of these roots and tubers in non ruminant feeds is generally low. This level of utilization is attributed to the low protein and dry matter and the potentially toxic cyanogenic glycosides in fresh cassava and irritating substance in cocoyam. Processing techniques such as fermentation, soaking, boiling, ensiling and sun-drying are adopted to remove the deleterious substances, and effects on animals. The comparable performance of pigs and poultry fed varying levels of roots and tubers and their by-products with those maintained on maize showed that they can be used as substitutes in non-ruminants diets at certain levels without detrimental effects. To achieve increase in the use of these root crops and their by-products for maize replacement in intensive non-ruminant production systems, adequate protein supplementation and proper processing are essential.
Keywords: By-Products, Cassava, Cocoyam, Non-Ruminants, Sweet Potato
Article Outline
1. Introduction
- The greatest proportional cost in livestock production is expended on feeding, with the exception of ruminants whose feed is based on pasture. In non-ruminant animals such as pigs and poultry, feed ingredients represent 65 to 70% of the total cost in an intensive production system in Nigeria as in many developing countries[1]. Energy source constitutes between 45 and 60 percent of finished feeds for these animals[2], and at present, maize is the commonly used source of energy in livestock feeds[3]. The increasing pressure on the use of maize by human population and livestock feed millers coupled with the cost of maize which fluctuates with the time of the year, thus making the cereal grain to be either scarce or expensive, stimulate the use of alternative sources of energy that are locally available, particularly the starchy roots and tubers that abound in many areas of humid tropics. In addition, their by products such as peels, vines and leaves are non competitive feed materials that can be developed as components of poultry and pig feeds. Although roots and tubers are cheap sources of energy, the extent of their practical use in non ruminant feeding has been limited. For example in Nigeria, 5% of total cassava production is used as feed. The presence of toxic cyanogenic glycosides and other undesirable substances, dustiness of the dried products, mouldiness during processing and the high fibre of the peel account for the low utilization in non ruminant production.[4, 5].Recently, emphasis has been placed on the expanded programme of cassava, sweet potato and cocoyam cultivation, and many high yielding varieties of cassava have developed and released through the improvement efforts of International Institute of Tropical Agriculture (IITA) and other collaborating institutions, suggests that production in excess of direct human consumption will become available for feeding farm animals in Nigeria. This paper reviews the potential value and constraints to increase use of these roots and tubers and their by-products as dietary supplements for non ruminants and fish. Additionally, this paper refers to ways that could increase the use of these feed sources.
2. Production and Nutritional Value of the Roots and Tubers
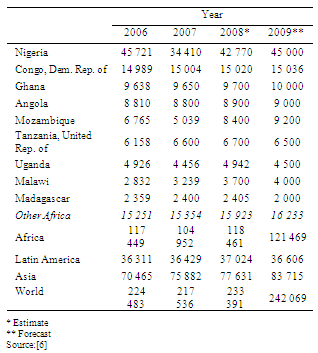
- Cassava, sweet potato and cocoyam are cultivated as staple food crops. They are efficient in producing cheap food energy. More than 228 million tons of cassava was produced worldwide in 2007, of which Africa accounted for 52%. In 2007, Nigeria produced 46 million tons making it the world's largest producer (Table 1). The yields of sweet potato was 15-20 tonnes per hectare and cocoyam give 25-30 tonnes per hectare of corns depending on planting density.
|
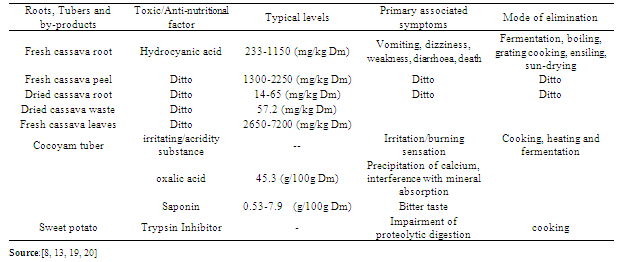
3. Antinutritional Factors
- Foods are complex substances that contain many chemical compounds, more than 50 of which are required to nourish the body. These nutrients include water, protein, lipid, carbohydrate, minerals and vitamins. Most plant foods consist of natural compounds or anti-nutrients that appear to function generally in defense against herbivores and pathogens. Anti-nutrients are potentially harmful and give rise to a genuine concern for human and animal health in that they prevent digestion and absorption of nutrients. They may not be toxic as such, but can reduce the nutritional value of a plant by causing a deficiency in essential nutrients or preventing thorough digestion when consumed[14]. The most commonly known and studied anti-nutritional factors in roots and tubers include cyanogenic glycosides, saponin, phytate, oxalate, enzyme inhibitors and total alkaloids (Table 3). They must be inactivated or removed before they are suitable for non ruminants or fish.[15, 16].The cyanogenic glycosides or cyanogens are glycosides of 2-hydroxynitriles that are synthesized and stored in cassava and are widely distributed among plants[17, 18]. The peel component has the highest concentrations of cyanogenic glycosides in cassava (Table 3). In cocoyam tubers, Abdulrashid and Agwunobi[11], Olajide, et al[13] reported wide variation (2.10 – 17.13mg/100g) in the level of these undesirable substances. Hydrolysis of cyanogenic glycosides releases hydrogen cyanide (HCN), which inhibit several enzyme systems, depress growth through interference with certain essential amino acids and utilization of associated nutrients[2, 21]. Cytochrome oxidase, is the primary site of action for ingested cyanide, an effective inhibitor of many metalloenzymes[22].The enzyme cytochrome oxidase in the mitochondria of cells is inactivated by hydrogen cyanide binding to the Fe2+/ Fe3+ contained in the enzyme. This results in a reduction of oxygen usage in the tissues[23] and oxygen starvation at cellular level, due to the effects of cyanide poisoning, resulting in death. Respiratory failure is therefore the cause of death since the respiratory centre nerve cells are extremely sensitive to hypoxia[24]. Other diseases associated with dietary cyanide intake include (i) konzo (Cliff et al 1997), a paralytic disease; (ii) tropical ataxic neuropathy (TAN)[25], a nerve-damaging disorder that renders a person unsteady and uncoordinated; (iii) goiter and cretinism[26]. Sheeba and Padmaja[21] observed that the palatability and shelf-life of cassava products may be prolonged by processing. The levels of cyanogenic glycosides and hydrogen cyanide are also reduced to safer limits by processing (peeling, slicing, boiling, fermentation) before consumption[27, 28].
|
|
4. Use of Cassava in Diets for Poultry
- Poultry feed constitutes more than 90 percent of all commercial livestock feeds produced. The use of cassava as a substitute for maize will therefore make its greatest impact if it can be incorporated into commercial poultry feeds. Certain precautions need to be taken to achieve satisfactory performance of stock on cassava-based diets. These include removal of cyanide, higher protein supplementation with fish meal, soybean, groundnut cake or methionine in its pure form and the prevention of microbial activity during sun-drying as well as overcoming dustiness. Most of the present studies indicate that satisfactory growth response has been obtained for growing chicken at 10% incorporation of cassava flour (lafun) or cassava peel into the diet; 40% inclusion of cassava flour or 20% inclusion of cassava peel in layer’s diet is satisfactory for egg production[2]. Combination of cassava root and leaves in ratio 4:1 could replace maize in poultry diets and reduce feed cost without a loss in weight gain or egg production[42]. Feeding cassava chips supplemented with Moringa oleifera leaf meal at 5 and 10% levels showed that cassava chips replacing maize at 55.56 and 83.33% in the diets of broilers has no negative effect on productivity and haematology when 5% Moringa oleifera leaf meal was added[43].
5. Use of Cassava in Diets for Pigs
- Cassava can be fed to pigs either fresh or parboiled (as commonly practice in small and medium scale pig farms), or included in the diets as a dried meal. Due to high level of moisture, fresh cassava is not recommended for feeding suckling and weaned pigs. Also, in starter pig diet, cassava meal should be limited because of the potential problems with palatability and its powdery nature which can affect respiratory organs. This can be overcome by pelleting. The use of cassava peels as a partial replacement for maize in young pig diets was cost effective and up to a 57% level of inclusion had no deleterious effect on the pigs[44]. Reports on growing-finishing pigs fed diets supplemented with groundnut cake, fish meal and brewer’s dried grains in which cassava meal or cassava peel substituted 60% or 100% of the maize respectively, showed no decline/adverse effects on performance and the economics of replacing maize with cassava or its by-product will depend on the price of protein supplementation that can provide an adequate margin of profit[45]. The authors concluded that 40% inclusion of cassava into growing pig diet was economically feasible. Ospina et al[46] found that pigs fed cassava root meal ad libitum, combined with 200g/day of crude protein throughout the growing-finishing period gave acceptable growth performance and carcass quality.
6. Use of Cassava in Diets for Fish
- The starch in cassava is highly digestible when compared to that of maize due to the high content of amylopectin[47]. Cassava can therefore be used as a source of energy in fish feed, but attention should be paid to the low protein, metabolizable energy and hydrogen cyanide (HCN) contents in cassava products. Studies on the use of cassava meal in fish feed[48 - 50] indicate that cassava can replace the conventional energy feed ingredients such as maize, broken rice and sorghum, which are commonly used in animal diet in most parts of Africa[51]. Cassava has been successfully used to replace maize in Clarias gariepinus fingerlings – 50% replacement[3]; C. garieipinus advanced fry[52] and hybrid catfish (Hetero X Clarias) – 66% replacement[53]. Inclusion of whole cassava root meal in the diet of fish enhanced growth and survival. Hence fish farmers can therefore take advantage of this ingredient as a replacement for more expensive maize when formulating feed for fish in aquaculture.
7. Use of Cassava Peel in Poultry Diets
- Cassava peel can be used to cut down the cost of production and lead to an active and sustainable development in livestock production[54]. Some workers[55 -57] recommended the inclusion of fermented cassava peel up to 15% in broiler diets with no adverse effects. For a reasonable performance of animals fed cassava-based diets, the rations must be nutritionally balanced and with protein source that contain sufficient sulphur-containing amino acids[58]. In the study on the influence of protein source on the performance and haematology of broiler chicken on cassava peel-based diets using fishmeal and groundnut cake, Egbunike et al[59] reported that broilers could be raised on cassava peel-based diets using groundnut cake as protein source without any adverse effect on performance indices. Also, Sogunle et al[60] studied the inclusion cashew nut reject and cassava peel meal in the diet of growing pullets and concluded that combination of 10% cassava peel meal and 30% cashew nut reject meal was appropriate for enhanced performance of growing pullets.
8. Potentials and Limitations of Cassava Leaves as Non-Ruminant Animal Feed
- Cassava, one of the world top calorie producers for human consumption, is generally grown without fertilization on soils with poor fertility and can survive prolonged water deficit[61], it is tolerant to acid soils, but the yield is limited by poor phosphorus (P) supply[62]. Cassava is planted mostly for its tuberous root, leaving the leaves to wither after harvesting the root. However, it is possible to obtain from cassava leaves more than 6 tonnes of crude protein ha-1 year-1 with proper agronomic practices directed toward foliage harvesting[5].Cassava leaf contains high level of crude protein, vitamins and nutritionally valuable minerals[63, 64]. The nutritional limitations of cassava leave include the HCN content, low digestible energy, bulkiness and possibly the high tannin content[65]. The inherent cyanogenetic glycosides may limit its use as a non ruminant feed. The cyanogenetic glycosides, is influenced by the nutritional status and age of the plant[66], and higher HCN levels were found in leaves from bitter than in sweeter varieties[67]. However, the HCN concentration and the bitterness associated with high cyanogenetic glycoside contents in leaves[68] decreases with the maturity of the leaves.The two most widely used processing methods are sun drying and ensiling. In the humid tropics, especially in the wet season, sun drying is difficult and may result in the production of low quality product with severe Aspergillus and aflatoxin contamination. Ensiling the leaves entails chopping into small pieces (2-3cm), then mix with additive and add common salt at 0.5% and store in sealed air tight plastic bags for two months could reduce HCN content up to 80% of the original concentrations. In poultry it was reported that broilers could tolerate diets containing 141 mg total cyanide kg-1 without any negative effects on growth performance[65]. According to Fasuyi and Aletor[69] cassava leaf protein concentrate can replace up to 60% fish meal without any negative effects on growth performance, haematology and serum metabolites of broiler starter. In pigs, the inclusion of 15% fresh cassava leaves in the diet had no adverse effects on the performance of growing-finishing pigs[70]. Dried or ensiled cassava leaves have been used at 16.5% and 20% respectively in the diets without significant effects on performance and carcass traits of growing pigs[71]. However, Ravindran[72] reported a depression in weight gain and feed efficiency when cassava leaf meals were included at up to 30% in diets for growing-finishing pigs. Khieu Borin et al[73] reported a significant improvement of daily weight gain of crossbred pigs fed a mixture of cassava leaves and water spinach as compared with those fed cassava leaves alone, due to increase intake and possibly the better amino acid balance of the mixture.
9. Use of Sweet Potato by Non-Ruminant Animals
- The sweet potato, (Ipomoea batatas) belongs to the morning-glory family Convolvuceace. It is cultivated primarily in tropical areas and ranked fifth among the most important food crop in the tropics[74]. The cost of production of sweet potato is much lower compared to cereal crops[75]. The carbohydrates of sweet potato are highly available and can be greatly utilized by non-ruminant animals[76]. Also, patatin which accounts for 30-40% of protein in the potato tuber is extremely well balanced, being nutritionally similar to casein[77].The leave meal has a high protein content of between 26 to 33%, with high amino acid score. It has good mineral profile and rich in vitamins like A, B2, C and E. Apart from its nutritive values, sweet potato leaves can be harvested many times throughout the year[78] thereby making the leaf meal to be abundant. The major factor limiting its use in monogastric animal is the presence of anti-nutritional factors[79]. The antinutritional substances present in the sweet potato leaves, according to Oyenuga[10], are the protease inhibitors and invertase. These substances can be inactivated by various processing methods like oven or sun-drying, boiling or steaming and grinding prior to inclusion in animal feeds.With regard to non-ruminant feeding, the data on sweet potato are limited. However, reports by Yeh and Bouwkamp[80] and Nwokolo[81] showed that up to 50% of the grain in corn-soybean diets could be replaced with sweet potato chips, without a significant depression in growth or production. With such high levels of replacement, it may be necessary to supplement such sweet potato – based diets with 0.2-0.5% of lysine. The dustiness of the sweet potato and high content of reducing sugars will tend to limit its use at such high levels in poultry and other non-ruminant ruminant diets[82]. Inclusion of sweet potato leaf meal in the diet of Tilapia zilli showed that levels up to 15% could be added without any negative effects on the growth, feed efficiency and protein digestibility[83]. In the same vein, Omoregie et al[84] revealed that Oreochromis niloticus could tolerate up to 15% level of inclusion of sweet potato peel.
10. Use of Cocoyam in Diets for Poultry
- Cocoyam is recognized as cheaper carbohydrate sources than grains or other tuber crops[85]. It has high caloric yield per hectare, low production cost[86] and relatively low susceptibility to insect and pest attack. Similarly, it is reported that cocoyam has readily digestible starch content because of its small particle size[87, 88]. It is almost competition-free with man in most places as it is eaten only as a last resort when a family can no longer afford garri or yam. It is therefore more likely to be available for use at lower cost. The use of cocoyam as food for man and animal has limiting factors such as storage and presence of antinutritional factors.Taro is closely related to Xanthosoma and Caladium, plants commonly grown as ornamentals. The antinutritional factors found in taro cocoyam include oxalates, phytates, Tannins and Saponins.[4]. However, some may serve as defensive mechanism against pests and diseases. Therefore oxalates have been found to be as defense mechanism and a storage reserve for calcium[89]. There is limited reference work on the utilization and inclusion of taro cocoyam as an alternative energy source in poultry production. However, Anigbogu[90] and Abdulrashid and Agwunobi[11] reported that taro meal should not exceed 25% replacement of maize in broiler diets. Tania (Xanthosoma sagittifolium) is a high-yielding, disease resistant crop. Its energy content appears moderate when compared with maize. However, like most varieties of cocoyam, the problem with Xanthosoma sagittifolium is its content of some antinutritional factors which could be a limitation to its use[91]. This limiting factors can be removed by boiling or sun-drying[92]. Esonu[93] reported that starter broilers could tolerate up to 20% inclusion levels of wild variegated cocoyam (Canadium hortulanum). On the other hand, Uchegbu et al[94] showed that raw sun dried cocoyam meal can be used in the diet of finisher broilers up to 15% inclusion level without being detrimental to their performance. However, 10% cocoyam inclusion level is the best in terms of daily weight gain, feed conversion ratio, and cost effectiveness.Wild cocoyam is a high moisture tuberous rootstock. Presently, it is not directly consumed by man and equally of no industrial use. Available literature on the feeding of wild cocoyam meals to finisher broilers suggests that it is a satisfactory energy ingredient at up to 20 % of the whole ration[95, 96]. However, its liberal use in monogastric animal feeding could be encumbered by the presence of some anti-nutritional factors (tannin and trypsin inhibitor), which adversely affect protein and energy utilization in broilers[96, 97]. The use of heat to inactivate these anti-nutritional factors could increase the use of wild cocoyam as a feed component in broiler diets.
11. Use of Cocoyam in Diets for Pigs
- Cocoyam corms intended for utilization as pig feed need to be cooked prior to drying and feeding to ensure removal of the toxic substance (oxalic acid) present in the corms, leaves and petiole of the plant. There are few actual experiments conducted on cocoyam utilization. However, it is recommended that cooked dried cocoyam can be fed on sows in gestation and late lactation but not to starter pigs or those in the early grower phase. Cocoyam silage may be fed to pigs in the growing and fattening stages, as well as to gilts in gestation and lactation. At levels of 20 – 40% of dry matter, cocoyam silage supported adequate growth rate in young pigs[93]. Ohaemenyi[98] reported that Xanthosoma sagittifolium corms can be cooked and used to some extent in the diets of growing pigs.
12. Conclusions
- Considering the chemical and nutritional characteristics of available alternative energy supplements (roots, tubers and their by-products), they have potential for increase use as alternative energy supplements for non ruminant production. Furthermore, these materials possess readily digestible energy. The comparable growth performance of pigs and poultry fed varying levels of roots, tubers and their by-products with those fed maize showed that they can be used as substitutes in non-ruminants diet at certain levels without being detrimental to their performance. However, for improved performance of animals, rations containing roots, tubers or their by-products must be formulated to contain good protein source and sufficient sulphur-containing amino acids. In addition, various processing methods (e.g. drying, boiling, frying, fermentation and ensiling) can be employed to eliminate or reduce the anti-nutritional factors present; this will improve the quality and safety of these feed materials. If these findings are diffused and adopted by farmers and livestock feed manufacturers, the amount of roots and tubers used in non-ruminant feed production in Nigeria would increase with consequent reduction in pressure on demand for cereal grains.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML