-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2012; 2(2): 6-11
doi: 10.5923/j.food.20120202.02
Effect of Galactomannans and Low Esterified Pectin Combinations on Fruit Preparation Synersis, Rheology and Stability on Storage
Omprakash H. Nautiyal
India malt Pvt. Ltd., Vill. Savli, Taluk Manjusar, Vadodara, Gujarat, 371995, India
Correspondence to: Omprakash H. Nautiyal , India malt Pvt. Ltd., Vill. Savli, Taluk Manjusar, Vadodara, Gujarat, 371995, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
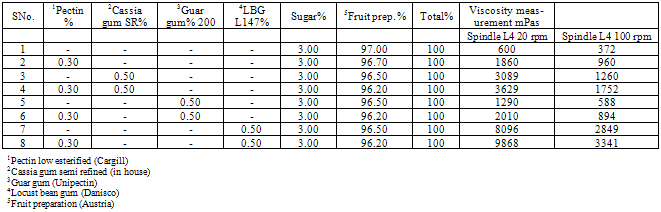
The objective of the study was to evaluate the fruit preparation syneresis, rheology and stability of the various blends of galactomannans. Percent composition of low estreified pectin (0.3%), semi refined cassia gum (0.50%) guar gum (0.50%), locust bean gum (0.50%) by w/v were changed keeping the sugar consistent. Fruit preparations were heated at 40oC for mixing of dry blends. Finally the blend was heated at 85oC and the end temperature was hold for 5 minutes. It was then cooled down to below 70oC, and evaporated water was added. It was cooled to 20oC and the viscosity was measured next day and after one week at 20 and 100 rpm. The blends were stored in freeze and viscosity was measured at 20oC. The synersis was found to be satisfactory without any cream formation at the top of the blends. The enhancement in the values of the viscosity may be attributed to the arrangement of galactose: mannose series arrangement in the respective galactomannans and plays important role in the stabilization of the food formulations.
Keywords: Low Esterified Pectin, Cassia Gum SR, Guar Gum, Locust Bean Gum, Synersis And Stability
Article Outline
1. Introduction
- Hydrocolloids are either linear or branched. Most are linear, meaning they are composed of one long chain with small side chains. Branched hydrocolloids, composed of many branches joined together in a bushy shape, include tree gums such as gum Arabic and the starch amyl pectin. Counter intuitively, branched hydrocolloids typically display lower viscosities (lower viscosity means thinner; higher viscosity means thicker) than linear hydrocolloids of the same size. Branched hydrocolloids are compact, acting like tennis balls, while linear hydrocolloids act like a tangle of spaghetti moving about in solution, taking up more space. For a given hydrocolloid, larger molecules having more sugar units will produce higher viscosities.The main reason for adding a gum or hydrocolloid to a food product is to improve its overall quality. This improvement may relate to its appearance, convenience, stability, cost, texture, etc., all of which are eventually judged by the consumer. The consumer usually judges the product by simply eating it and seeing how it tastes. At this point he does not care if the added gum is a galactomannan or a sulphated galactan, nor does he worry about whether the vitamin A in the product is naturally derived from sharks liver or synthesized from b ionone. He is concerned with the taste and mouth feel of the product. If it tastes good, he is happy if it tastes bad, he is unhappy, and so eventually is the manufacturer of that specific food item. Viscosity is the resistance to flow of a liquid system. In colloidal suspensions it is increased by the thickening of the liquid phase as a result of liquid absorption and consequent swelling of the dispersed colloid. This thickening, or viscosity producing, effect of gums in food products is, in turn, responsible for other functional effects such as the suspension of solid particles, the emulsification of oil and water phases, the stabilization of liquid solid gas phases, the dispersion of solid and liquid phases, and related phenomena. When hydrocolloids are used as viscosity producing agents for the purpose of suspending, emulsifying, or stabilizing a food system, shelf stability is extremely important and the selection of the proper hydrocolloid is critical. Degradation of the hydrocolloid and the resulting reduction in the viscosity of polymer solutions may impair the flow properties and appearance of the product sufficiently to reduce its consumer acceptability.1The quality criteria of fruit preparations are mainly de-termined by their rheological flow behaviour. The formula-tion of the fruit preparation as well as the thickening agents used has a decisive effect on the flow behaviour. The mixing behaviour with the milk product and the syneresis behaviour also affect the quality of yoghurt fruit preparations. Pectins for fruit preparations, such as Low Methyl ester Classic Apple Pectins and Low Methyl ester Amidated Pectins from H&F (Figure 1), are systematically standardized to specific flow behaviour. Thus if the appropriate pectin is selected for a pre-determined formulation, optimum products are ob-tained.1, 2-3
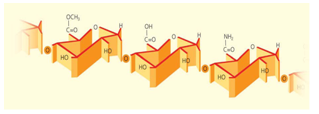 | Figure 1. Molecular structure of esterified pectin |
2. Materials and Method
- Low esterified pectin, Locust bean gum and Fruit concentrate were purchased from the international suppliers. Guar gum was purchased from Shriram Hydrocolloids Corporation. Semi refined cassia gum was manufactured in house. Sugar was purchased from India.Dry ingredients were mixed and pre blend was prepared. The fruit preparation was heated to 40℃. Pre blend was dispersed into the fruit preparation. It was then heated up to minimum 85℃. When the time was found too long the heating was stopped at 85℃ and the end temperature was noted. The lumps formation was observed during dispersion of pre blend. The heating was further hold for 5 minutes and then allowed to cool down below 70℃. The evaporated water was compensated. It was finally cooled down to 20℃.The viscosity was measured next day and after one week at 20 and 100 rpm. The blends were stored in the freeze and were measured at 20℃. The stability test of the prepared blend was tested after one week. The blends were stored in the freeze at < 80℃. The temperature was monitored regularly and assured to be at aforesaid temperature6, 7Since most gums are long chain polymers, they are subject to the type of molecular breakdown caused by cleavage of molecular bonds, resulting in lower viscosities. Determination of the exact causes of degradation or loss of viscosity is often difficult. Frequently, polymers are degraded by the use of high shearing equipment used to put them into solution, or by the high temperatures used in processing. In general, the low viscosity natural gums are more stable than the high viscosity types. Studies on the comparative stabilities of gums are more valid if comparisons are made between solutions of equal viscosity rather than of equal concentrations, which is so often the case.
2.1. Stability Tests after Week
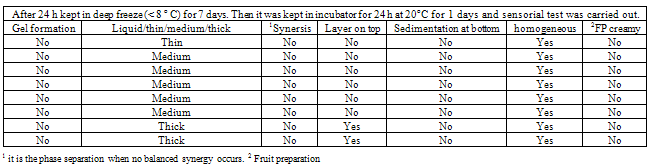
- The blends were evaluated for synersis, sedimentation at the bottom, layer on the top of the blends; creaminess of the blends (may contain destabilized particles). Most important was homogeneous phase observation. Seldom do processors immediately think of gums when working with fluid products. The truth is, however, that they can be helpful in these products at low concentrations. Most obviously, the viscosity of flavoured milk can be increased and maintained through the addition of a gum. Less obvious, is the stabilizer's ability to maintain particulates in suspension.Flavoured milk products contain sweeteners and flavouring agents such as cocoa powder. More often than not, these ingredients are not dissolved, but merely suspended. Eventually, gravity causes them to settle out.At low concentrations, a stabilizer can help keep the ingredients suspended without making the product too thick. This works in two ways:First, a stabilizer, such as starch, builds viscosity making it take longer for gravity to pull the particles out of the suspension. Second, if a gum like carrageenan is used, a colloid network exists even though the concentration is low enough to prevent actual gelling. This network supports particulate matter and prevents settling.Working on the frost lineDistribution and handling exposes hard frozen products to a wide variety of environmental and temperature changes. This exposure to temperature fluctuation is known as "heat shock".8-9
3. Results and Discussion
- The study was firstly carried out blending 97% of fruit concentrate and 3% of sugar and on testing its viscosity at 20 rpm and 100 rpm, (Table 1 and 2) it was found to be 600 and 372 mPas. The preparation did not form gel. It was thin and no synersis was found occur. Neither layer was found on top of it or the sedimentation at the bottom. It was observed to be homogeneous and no cream formation occurred. 0.50% of cassia gum SR when mixed with 96.50% of fruit concentrate viscosity of the blend was comparatively high at 20 rpm than that at 100 rpm. But when 0.30% of low esterified pectin along with 0.50% cassia gum SR was mixed with 96.20% of fruit concentrates the viscosity of the blend was appreciably high at both the rpm. Locust bean gum as alone and while mixed with 0.3% of LEP with 96.50 and 96.20% fruit preparation found to exhibit the highest viscosities during entire study of the blend preparation. Though the use of LBG alone and/or with LEP found to yielding thicker blend without any synersis, and remain homogeneous but with the cream formation on the top of the blends. As observed from the table preparations from 2-6 found to yielding the blends with medium phase behaviour and without synersis, sedimentation, layer on the top. They were remained homogeneous even after a week and no cream formation was resulted.
|
|
|
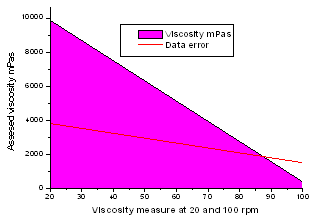 | Figure 2. Rheological behaviour of fruit preparation with galactomannans |
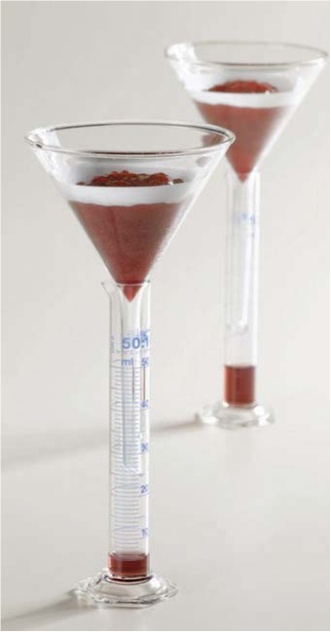 | Figure 3. Synersis test for fruit preparations |
4. Conclusions
- The filling temperature has an important influence on both the texture and the firmness of the final product. If a product, e.g. a fruit preparation, is produced and deposited, elastic gels of constantly high gel strengths are obtained as long as the filling temperature exceeds the setting temperature of the respective fruit preparation. If the filling temperature decreases, and, finally drops below the setting temperature, pre-gelation starts and part of the overall reachable gel strength is lost. At the same time, the lower the selected filling temperature, the more the viscosity of the gel texture of the pre-gelled fruit preparation increases. The enhancement in the values of the viscosity may be attributed to the arrangement of galactose: mannose series arrangement in the respective galactomannans and plays important role in the stabilization of the food formulations.Many hydrocolloids gel when cooled. Sometimes these gels can be melted again, such as gelatin, and sometimes they cannot, such as the pectin in a jam. Methylcellulose forms a gel when heated that melts on cooling. Some thermally reversible gels show temperature hysteresis, that is, the setting temperature of the gel is lower than the temperature needed to melt the gel. This property can be very important to a chef. For example, agar sets around 35°C but melts at around 90°C. The low set temperature makes agar easy to work with, and the high melt temperature allows agar preparations to be served hot. Thermally formed gels can also be slow set or snap set. Snap setting hydrocolloids, like gellan, gel instantly below their gelation temperature. As a rule of thumb, gelling hydrocolloids and thickening hydrocolloids can often be mixed to get the benefits of both (locust bean gum can be added to kappa carrageenan to give it a better texture, for example) without synergistic effects that will damage a recipe. Charged and uncharged hydrocolloids can also often be mixed without incident, like methylcellulose and alginate (for more information on synergistic effects.11, 12
Notes
- 1The research was conducted from business point of view so that the in house Cassia gum along with outsourced hydrocolloids may find greater commercial application. It is well known that hydrocolloids are in great demand worldwide in the application of stabilizing the food from synersis and rheology point of view. The study was found to be useful employing the fruit preparation from Austria
ACKNOWLEDGEMENTS
- I am thankful to Mr. Peter Huber as Food technologist for his help in evaluating the fruit preparation and providing fruit preparation for the research from Austria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML