Abdel Moneim E. Sulieman 1, Waleed A. Mustafa 2
1Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
2Department of Food Science and Technnlogy, Faculty of Agriculture, University of Bakht Al-Ruda, ElDueim, Sudan
Correspondence to: Abdel Moneim E. Sulieman , Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
In the present study the prevailing drying methods for fish preservation in ElDueim area, central Sudan were assessed. The processes and analysis were done in two seasons: summer and winter. The chemical analyses indicated the similarity of most of the tested chemical components of the various samples. High microbial load was detected in raw fishes indicating that their quick spoilage at ambient temperature, and the presence of coliforms, staphylococci and salmonella indicates that the raw fish handling is not safe. On the other hand, most of the examined dried fish products were devoid of pathogenic bacteria, and sharp decrease was observed in the numbers of indicator microorganisms like coliforms. The sensory analysis indicated higher acceptance of most of the tested samples.
Keywords:
Drying, Chemical Composition, Salmonella, Microbiology
Cite this paper:
Abdel Moneim E. Sulieman , Waleed A. Mustafa , "Quality Characteristics of Dried Fish Obtained From Eldeim Area, Central Sudan", International Journal of Food Science and Nutrition Engineering, Vol. 2 No. 1, 2012, pp. 1-6. doi: 10.5923/j.food.20120201.01.
1. Introduction
Processing of fish involves primarily the application of preservation techniques in order to retain quality and increase shelf life. It may also mean adding value to produce a wide variety of products. A number of methods are used to preserve fish. There are various techniques based on temperature control, using ice, refrigeration or freezing; others on the control of water activity that includes drying, salting, smoking and freeze-drying.Dry Fish is described as any fishes which had developed a strong odour within hours of capture and salted for about four days and then dried. It is highly salted and semi-dried fishery products with an obnoxious odour and a cheesy but rich fishy flavour widely liked as a sea food item worldwide. Traditionally, dried fish represents a low cost source of high quality protein[1]Sudan has immense fisheries resources within its inland waters especially along the River Nile and in the marine sub-sector along the Red Sea coast. Estimates suggest that fish production could double and still be short of the full potential. Whilst techniques and vessels used in the inland fishery remain artisanal in nature, participants in the fisheries are becoming increasingly commercialized and investors are now operating fleets of simple vessels. Sudan is, however, a significant importer of fish from other areas of the Great Lakes region of Africa. Most experience with aquaculture has been along the coast with pearl culture but increasinglyinvestors are establishing catfish and tilapia pond farms.Fish processing in carried out in Sudan by traditional methods, so the amounts of the processed samples is relatively few with a short shelf life and consumed locally. Therefore it is of prime importance to encourage upgrading of fish industry and to increase the investment in this sector, and to make use of stored fishes commercially via methods of preservation and processing of fishes.The objectives of the present project include: evaluation of the prevailing drying methods of fish and an attempt to upgrade these methods following good manufacturing practices techniques.
2. Materials and Methods
Samples of fresh fish, namely: garmout, bolti, kas, kabarous, kharsha and amokoro were which were collected from Dueim market (Central Sudan) on White Nile, from anglers immediately after landing, during two seasons summer and winter (2010). In addition, processed samples were collected from small-scale producers who producing their products traditionally. These samples were collected in sterile polyethylene bags. The samples were then transported in strict sterilization conditions to the laboratory where microbiological and chemical analyses were immediately carried out.The fish and dried fish products received for analysis in the laboratory were cleaned without washing to remove adhering matter. The smaller fishes were passed through the meat mincer in the intact form. However, in case of larger fishes, only meat was taken for analysis. For this, the head and fins were removed and the body was cut along the abdomen. All the viscera including the gonads were removed. Then the fish was cut along the back and the backbone and as far as possible all the ribs were removed and the meat and fat were carefully cleaned of skin.Drying process The fish drying started after preparation. After gutting the fish, it was either dried whole, or split along the spine leaving the tail connected. The fish was hung on the flakes from March -June (ideal time; i.e. summer). The fish flakes were subjected to vacuum packaging. After two months of hanging on the flakes, the fish was then matured for another month indoors in a dry and airy environment. During the drying, about 85% of the water in the fish disappeared.Chemical methodsThe proximate analysis was carried out in triplicates in all samples according to AOAC[2], these analyses included: the contents of moisture, ash, protein, fat, crude fibre and carbohydrates. The pH values were determined according to AOAC[3] methods using a digital pH meter.Microbiological analysisFor the microbiological analysis[4], appropriate dilutions of the respective fish and dried fish samples in 0.1 gm aliquots were spread on pre-poured plates of Plate count agar (PCA) for enumeration of total viable count, Mac Conkey agar for coliforms, Baird-Parker agar (BPA) for staphylococci spp. and Potato Dextrose Agar (PDA) for yeasts and moulds. The PCA and PBA plates were inoculated at 37℃ for 24-48 h, while PDA plates were incubated for 72 h at 25℃. Characteristic colonies appearing on the respective selective agar media were counted, multiplied by the dilution factor and expressed as colony forming units per ml c.f.u/g.For Salmonella detection, ten grams of sample were weighted aseptically and mixed well with 100 ml sterile nutrient broth. This was incubated at 37℃ for 24 hours. Then 10 ml were drawn aseptically and added to 100 ml selenite broth. The broth was incubated at 37℃ for 24 hours. Then with a loopful streaking was done on dried Bismuth sulphite agar plates. The plates were then incubated at 37℃ for 72 hours. Black metallic sheen discrete colonies indicated the presence of salmonella. A confirmatory test was carried out by taking a discrete black sheen colony and subculturing it in a Triple sugar iron agar tubes. Production of a black colour at the bottom of the tube confirms the presence of salmonella.Sensory evaluationDried fish samples were subjected to panel tests. The performance of judges towards these products was tested using hedonic scale, whereby 15 panelists were selected each time. The samples were presented so that each sample had an equal chance to be tested first, second or last. The result obtained by the panelists was converted to scores ranging from like extremely (9) to dislike extremely (1)[5]. Statistical analysisThe data were subjected to statistical analysis using analysis of variance. Mean separation was done according to Duncan’s Multiple Range Test at 5% level.
3. Results and Discussion
The chemical composition, pH and energy values of the various fish types collected from anglers at El Duiem coast during the first season, summer are presented in Table (1). The contents of moisture, protein, ash, fat, crude fibre and carbohydrates varied considerably and ranged between 51.0-55.0 %, 18.5-21.5%, 12.9-14.2%, 7.7-8.9%, 0.9-1.8% and 2.6-6.2%, respectively. The pH value ranged between 5.5- 6.3, and energy varied greatly between the various samples (ranged from 385 to 670 kJ). On the other hand, during the second season, winter, the contents of moisture, protein, ash, fat, crude fibre and carbohydrates ranged between 48.7-54.8 %, 18.8-23.4%, 12.7- 14.5%, 7.7-8.7.2%, 0.9-1.2% and 3.8-6.3%, respectively. However, the pH value ranged between 5.6- 6.3, and energy varied greatly between the various samples (373-630 KJ). The protein contents determined in this study were within the standard values which is usually somewhere between 15 and 20 per cent, but values lower than 15 per cent or as high as 28 per cent are occasionally met with in some species[6]. Fish protein provides a good combination of amino acids which is highly suited to man’s nutritional requirements and compares favorably with that provided by meat, milk and eggs. The fat content of fish can vary very much more widely than the water and. There is usually considerable seasonal variation in the fat content of fatty fish; for example a starved herring may have as little as ½ per cent fat, whereas one that has been feeding heavily to replenish tissue may have a fat content of over 20 per cent. Sardines, sprats and mackerel also exhibit this seasonal variation in fat content. As the fat content rises, so the water content falls, and vice versa; the sum of water and fat in a fatty fish is fairly constant at about 80 per cent[6].The amount of carbohydrate in white fish muscle is generally too small to be of any significance in the diet; hence no values are given in the tables. In white fish the amount is usually less than 1 per cent, but in the dark muscle of some fatty species it may occasionally be up to 2 per cent. The composition of a particular species often appears to vary from one fishing ground to another, and from season to season, but the basic causes of change in composition are usually variation in the amount and quality of food that the fish eats and the amount of movement it makes.Tables 3 and 4 show the chemical composition of dried fish samples prepared under controlled conditions using various types of fish during the fist season (summer) and second season (winter), respectively. The contents of moisture, protein, ash, fat, crude fibre and carbohydrates (Table 3) ranged: 4.3- 5.1, 50.0-52.2, 28.2-32.2, 6.7-8.0, 1.0-1.6 and 3.3-8.8%, respectively. The pH and energy values ranged 5.8-6.5 and 450-600 KJ, respectively. On the other hand, contents of moisture, protein, ash, fat, crude fibre and carbohydrates during winter (Table 4) ranged: 4.1-5.0%, 48.6- 53.0%, 28.0-32.9%, 7.1-8.8%, 1.1-1.6% and 3.3-9.4%, respectively. The pH and energy values ranged 5.8-6.5 and 455-625 KJ, respectively. Azam et al.[7] has also reported similar protein, fat and ash contents of 10 species of dried fish.Drying is a common practice in meat, fish and other animal protein based industry, because it preserved the quality for an extended time and offers several advantages such as insignificant alterations and minimum deterioration in the product.The moisture content seems to be an inexact indicator of the susceptibility of a product to undergo microbial spoilage. A major factor, which determines the microbial, chemical and enzymatic stability of foods, is the water activity (Aw)[8].The processor, the nutritionist, the cook and the consumer all have a direct interest in the composition of fish. The processor needs to know the nature of the raw material before he can apply correctly the techniques of chilling, freezing, smoking or canning. The nutritionist wants to know what contribution fish can make to the diet and to health, and the cook must know for example whether a fish is normally lean or fatty in order to; prepare it for the table. The consumer is interested not only in whether a particular fish tastes good, which is a matter of opinion, but also in whether it is nutritious. While the consumer is interested mainly in the edible part of the fish, that is the flesh or muscle, the fish meal manufacturer is concerned with the composition of the whole fish, and the processor of fish oils wants to know what is in the liver. Measurement of constituents of fish products is sometimes necessary to meet specifications or to comply with regulations. For example, the fish content of fish cakes or the oil content of fish meal may need to be known in order to meet certain commercial or legal requirements. Fish is one of the most valuable sources of high grade protein available to man in this hungry world, and knowledge of its composition is essential if the fullest use is to be made of it. Table 1. Chemical composition, pH and energy values of fish samples collected from anglers at EDuiem coast (during summer)
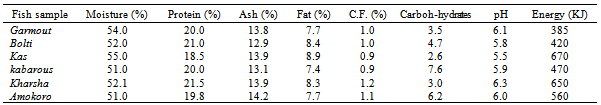 |
| |
|
Table 2. Chemical composition, pH and energy values of fish samples collected from anglers at EDuiem coast (during winter)
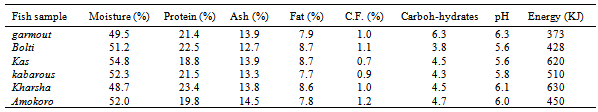 |
| |
|
Table 3. Chemical composition of dried fish samples (during summer)
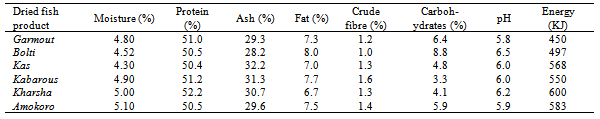 |
| |
|
Table 4. Chemical composition of dried fish samples (during winter)
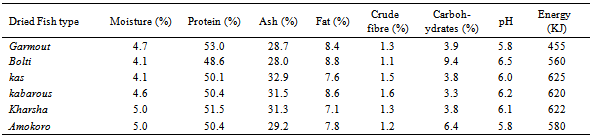 |
| |
|
The main constituent of fish flesh is water, which usually accounts for about 80 per cent of the weight of a fresh white fish fillet. Whereas the average water content of the flesh of fatty fish is about 70 per cent, individual specimens of certain species may at times be found with a water content anywhere between the extremes of 30 and 90 per cent[6].The results of the microbiological analyses included the load of bacteria, yeasts and moulds and pathogenic microorganisms in various samples of 10 different species of fish during the first season, summer and the second season, winter, are shown in Tables 5 and 6, respectively. Highest total viable count of bacteria (5.1 x 106 cfu/g) was observed in garmout and lowest (2.3 x 105 cfu/g) in kas during summer. While in winter, the highest total viable count of bacteria were observed in garmout fish (2.2×106 cfu/g) and the lowest (8.6×10 3) in amoroko fish. The other fish types had almost similar results. Yeast and mold count was the highest (7.4×103) in kas during winter and lowest (7.0 x 101 cfu/g) in khasha during summer. The other fish types had almost similar results. Total coliform bacteria was detected in all the fish samples. Coliform count measured by most probable number (MPN) method was ranged 100 to 240/g. Eschrichia coli was not detected in all examined fish samples during the two seasons. Highest staphylococci count was observed in bolti (3.8×103 cfu/g) during winter, while the lowest (1.2× 102 cfu/g) during summer. Salmonella was detected in about 60% and 70% of the fish samples analyzed during summer and winter, respectively. High microbial load in raw fishes indicates that raw fish would decompose very quickly at ambient temperature, and the presence of coliforms, staphy- lococci and salmonella indicates the raw fish handling is not safe. Fish is thus a product that needs proper handling and processing in order to preserve nutrients and its functional components that promote good health.| Table 5. The Microbiological Characteristic of fresh fish collected from anglers at ElDueim coast (during summer) |
| | Fish type | Total viable count of bacteria (cfu/g) | Coliform MPN per gram | Yeasts and moulds (cfu/g) | Staphyloc-occi | Detection ofSalmonella | | Total | E. Coli | | Garmout | 5.1 x 106 | 240 | 0 | 2.8 x 102 | 1.4×10 2 | - ve | | Bolti | 2.7×10 6 | 240 | 0 | 5.5 x 102 | 1.2×10 2 | +ve | | Kas, | 2.3 x 105 | 240 | 0 | 1.5 x 102 | 7.3×10 2 | -ve | | Kabarous | 3.3×105 | 120 | 0 | 8.6 x 102 | 4.7×10 2 | +ve | | Kharsha | 8.5×105 | 100 | 0 | 7.0 x 101 | 3.3×103 | +ve | | Amokoro | 7.2×105 | 240 | 0 | 4.5 x 102 | 4.5×10 2 | -ve |
|
|
| Table 6. The Microbiological Characteristic of fresh fish collected from anglers at ElDueim coast (during winter) |
| | Fish type | Total viable count of bacteria (cfu/g) | Coliform MPN per gram | Yeasts and moulds (cfu/g) | Staphyl-ococci | Detection ofSalmonella | | Total | E.coli | | Garmout | 2.2×106 | 100 | 0 | 3.6×10 3 | 2.2×10 2 | +ve | | Bolti | 8.3×10 5 | 240 | 0 | 5.3 ×10 3 | 3.8×10 3 | -ve | | Kas, | 3.5×105 | 120 | 0 | 7.4×10 3 | 5.3×10 2 | +ve | | Kabarous | 4.2×105 | 240 | 0 | 5.3×10 4 | 2.3×10 2 | +ve | | Kharsha | 6.4×103 | 180 | 0 | 1.8×10 4 | 2.3×103 | +ve | | Amokoro | 8.6×10 3 | 240 | 0 | 2.8×10 3 | 2.7×10 2 | -ve |
|
|
| Table 7. The Microbiological Characteristic of dried fish samples (during summer) |
| | Dried fish product | Total viable count of bacteria (cfu/g) | Coliform MPN per gram | Yeasts and moulds (cfu/g) | Staphylc-occi | Detection ofSalmonella | | Total | E. coli | | Garmout | 1.4×10 4 | 0 | 0 | 4.0×10 2 | ND | -ve | | Bolti | 6.7×10 4 | 0 | 0 | 5.6×10 2 | ND | -ve | | Kas, | 2.8×10 4 | 0 | 0 | 3.3×10 3 | ND | -ve | | Kabarous | 1.2×10 4 | 0 | 0 | 4.9×10 3 | ND | -ve | | Kharsha | 7.2×10 3 | 0 | 0 | 7.3×10 2 | ND | -ve | | Amokoro | 8.5×10 3 | 0 | 0 | 5.7×10 3 | ND | -ve |
|
|
| Table 8. The Microbiological Characteristic of dried fish samples (during winter) |
| | Dried fish product | Total viable count of bacteria (cfu/g) | Coliform MPN per gram | Yeasts and moulds (cfu/g) | Staphylc-occi | Detection of Salmonella | | Total | E. coli | | Garmout | 3.4×10 3 | 0 | 0 | 5.2×10 2 | ND | -ve | | Bolti | 5.6×10 3 | 0 | 0 | 4.7×10 2 | ND | -ve | | Kas | 3.9×10 4 | 0 | 0 | 3.2×10 3 | ND | -ve | | Kabarous | 2.4×10 4 | 0 | 0 | 4.7×10 3 | ND | -ve | | Kharsha | 7.2×10 3 | 0 | 0 | 5.3×10 2 | ND | -ve | | Amokoro | 8.5×10 3 | 0 | 0 | 4.8×10 3 | ND | -ve |
|
|
The results of the microbiological analyses of various dried fish samples produced during the first season, summer and the second season, winter, are shown in Tables 7 and 8, respectively. The highest total viable count was observed in dried bolti (6.7×104 cfu/g) produced during summer, and the lowest (3.4×103 cfu/g) in dried garmout produced during winter. All dried fish products were free from coliforms and staphylococci bacteria. However, the yeasts and moulds ranged from 1.2×102 to 5.7×103 cfu/g, with the amoroko dried fish (highest) and garmout dried fish (the lowest) during summer. Yeasts and moulds are examples of fungi, and moulds are responsible for the food spoilage and produce mycotoxin[9]. Similar trend of yeast and mould count was observed in samples produced during winter.In general, fish are risky commodities which are difficult to handle and distribute. However, if fresh fish is handled properly it will rarely loose its wholesomeness.The chemical analyses indicated the similarity of most of the tested chemical components of the fresh and dried fish products during the two seasons.The spoilage of fish and fish products depends on a number of factors. These factors as well as these spoilage mechanisms must be thoroughly understood before developing proper handling and pretreatment methods and preservation technologies for fresh, in addition to In general, microbiological analyses of fresh fish collected from anglers at El Dueim coast indicate that most of these samples were not safe microbiologically, and this could be a consequence of unhygienic conditions during handling and marketing. In contrast, the dried fish samples were safe microbiologically, however, indicator microorganism such as coliforms, and pathogens such as staphylococci and salmonella were devoid in all processed fish samples during the two seasons. | Table 9. Sensory evaluation* of some of dried fish products during summer |
| | Dried fish product | Appearance | Texture | Colour | Flavour | Overall acceptability | | Garmout | 6.9 b | 6.6 b | 7.3 a | 6.8 b | 7.4 a | | Bolti | 7.7a | 7.8 a | 7.3 a | 7.2 a | 7.5 a | | Kas | 7.7 a | 7.8 a | 7.4 a | 7.3 a | 7.6 a | | Kabarous | 7.8 a | 7.3 a | 7.2 a | 7.5 a | 7.5 a | | Kharsha | 6.8 b | 7.3 a | 6.9 b | 7.5 a | 7.1 a | | Amokoro | 7.1a | 7.0 a | 7.5 a | 7.1 a | 7.2 a |
|
|
Sensory evaluationIn the quality assessment of fresh fish the sensory evaluation is most important. As quality deterioration progresses, several off-odours can be noticed. Many different odour compounds can be perceived but some are having very low odour threshold values.The sensory evaluation results of the dried fish samples are shown in Table (9). The results show that there were insignicant differences in most of the sensory parameters of most of the dried fish samples. However, significant differences were recorded in appearance of dried fish products prepared from Garmout and Kharsha when compared with other dried fish products. The panelists gave high scores for most of the sensory attributes, and all the dried fish products were highly accepted by the panelists. However, dried Kas fish products were given the highest scores, while dried Amoroko fish products were given the least scores of acceptance.
4. Conclusions
The chemical analyses indicated the similarity of most of the tested chemical components of the fresh and dried fish products during the two seasons.The spoilage of fish and fish products depends on a number of factors. These factors as well as these spoilage mechanisms must be thoroughly understood before developing proper handling and pretreatment methods and preservation technologies for fresh, in addition to proper packaging and storage of the products.In general, microbiological analyses of fresh fish collected from anglers at El Dueim coast indicate that most of these samples were not safe microbiologically, and this could be a consequence of unhygienic conditions during handling and marketing. In contrast, the dried fish samples were safe microbiologically, however, indicator microorganism such as coliforms, and pathogens such as staphylococci and salmonella were devoid in all processed fish samples during the two seasons.
ACKNOWLEDGEMENTS
This work forms part of a research sponsored by the Sudanese Ministry of Higher Education and Scientific Research and carried out at Department of Food Science and Technology, Gezira University, Sudan. The authors express their sincere thanks to all staff members, techniques and the colleagues for their assistance.
References
| [1] | Souness, R. (1988). Reducing post harvest losses associated with dried fish production in Indonesia. Infosamak International, 8:38-40 |
| [2] | A.O.A.C .(2000). Official Methods of Analysis (17th edition) Association of Official Analytical chemists, Arlington, VA, USA |
| [3] | A.O.A.C. (1990). Official Methods of Analysis. Association of Official Analytical Chemists, Washington. D.C., USA |
| [4] | Harrigan W.F. and MacCance, M, E, (1976). "Laboratory methods in food and dairy microbiology". Academic Press. London, New York and San Francisco |
| [5] | Larmond, E. (1982). Laboratory Methods for Sensory Evaluation of Food. Research Branch, Canada Department of Agriculture, Publication 1936 Ottawa, Canada |
| [6] | (http://www.fao.org/wairdocs/2011) |
| [7] | Azam K, Ali MY (2004): Biochemical Assessment of selected fresh fish. J. Biol. Sci. 4(1): 9–10 |
| [8] | Troller JA & JHB Christian Water activity and Food. Academic Press, New-York, 1978: 9- 11 |
| [9] | Buchanan, R, L. & Doyle M. P. (1997). Food borne disease significance of Escherichia coli 0157: H7 and other enter hemorrhagic E.Coli .J. Food Technology. 51 (10): 69-76 |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML


