-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2022; 12(1): 1-8
doi:10.5923/j.ep.20221201.01
Received: Jan. 20, 2022; Accepted: Feb. 2, 2022; Published: Feb. 15, 2022

The Photoelectric Effect and Its Applications to Solar Cells
Krishiv Bhatia
Monta Vista High School, Cupertino, CA, USA
Correspondence to: Krishiv Bhatia, Monta Vista High School, Cupertino, CA, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The photoelectric effect occurs when electrically charged particles are released from or within a material when illuminated by light (or electromagnetic radiation). The light ejects electrons from the surface of the metal, and these electrons can cause an electric current to flow. The phenomenon was discovered in 1887 by the German physicist Heinrich Hertz. In 1905, Albert Einstein explained the photoelectric effect in a paper for which he won the Nobel Prize in physics in 1921. The photoelectric effect shows that light exhibits particle nature while the other properties like diffraction and interference indicate the wave nature of light. Hence, light behaves both like a wave and a particle. Hence, particles like electrons, protons, and even a soccer ball can behave like waves (although the wave properties are only observed at subatomic scales). This phenomenon is called wave-particle duality. The photoelectric effect has many applications ranging from image sensors, astronomy, photomultipliers, photoelectron spectroscopy, photocells (or solar cells), photocopiers, photodiodes, and phototransistors. The photocell is perhaps the most crucial application and is commonly found in solar panels. It works on the basic principle of the light striking the cathode, which causes the emission of electrons, producing current. The photomultiplier tube uses the photoelectric effect to convert light intensity into electrical currents.
Keywords: Photoelectric effect, Electromagnetic radiation, Albert Einstein, Wave-particle duality, Electron, Electric current, Photovoltaic, Quantum dots, Perovskites Solar Cells, Crystalline Silicon Solar Cells, Monofacial, Bifacial Solar Cells, Thin-Film Solar Cells, Organic Photovoltaics, Multijunction solar cells, Concentrated Photovoltaics
Cite this paper: Krishiv Bhatia, The Photoelectric Effect and Its Applications to Solar Cells, Energy and Power, Vol. 12 No. 1, 2022, pp. 1-8. doi: 10.5923/j.ep.20221201.01.
Article Outline
1. Introduction
- When light (or electromagnetic radiation) illuminates a metal, electrons are knocked-off from the surface of the metal, and these released electrons cause an electric current to flow. This phenomenon, called the photoelectric effect, is based on light consisting of tiny packets of energy known as photons or light quanta, which confirmed the wave-particle duality nature of light [1].The photoelectric effect has many applications. Perhaps the most critical application is the photocell, which is used in building solar cells. A photocell transforms light into electrical energy by producing voltage. As such, they can be used as sensors to detect light [2,3,4].A solar cell contains a semiconductor material which can be silicon. When light shines on the solar cell, it knocks off electrons from the semiconductor material's atoms and causes an electric current to flow -- that is, electricity. Multiple solar cells are soldered into circuit boards to form photovoltaic modules, and modules are combined to produce solar cell arrays to generate a more significant amount of electricity [5].
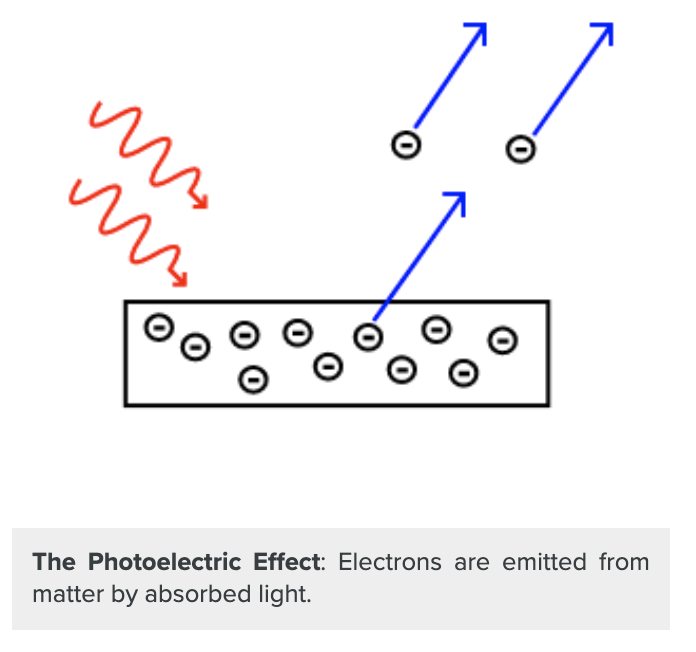 | Figure 1. The Photoelectric Effect [1] |
2. Research
2.1. The Photoelectric Effect
- In 1887, the German physicist Heinrich Hertz found that he could increase the sensitivity of sparking by illuminating it with UV light. Subsequently, J.J. Thompson discovered the electron in 1897 and attributed the increased sensitivity to light pushing electrons [1,2].However, the conclusion above did not fit with the classical theory of electromagnetic radiation, that is, light behaved like transverse waves. It was not clear until Albert Einstein said that light is a discrete quanta of energy or photons. According to Planck's formula, the energy of photons is proportional to their frequencies [1,2]:
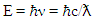 | (1) |
2.2. The Wave-Particle Duality
- The wave-particle duality refers to the principle that matter and light exhibit both particle and wave characteristics. This phenomenon is only detectable on atomic scales. Light can behave like a wave with a frequency determined by the particle's energy. Alternately, it acts like a particle with an energy proportional to the frequency of the wave [1]. From a quantum mechanics angle, each particle has associated with it a wave function 𝛹(x,t), such that |𝛹(x,t)|2 gives the probability of finding it at a point x at time t [1].
2.3. The Photocells
- A photocell is a sensor. It has a resistor whose resistance decreases depending on how much light falls on it. This phenomenon is called photoconductivity. They are used in light-sensitive detector circuits. Figure 2 shows the Advanced Photonix PDV-P5002 photocell [8].
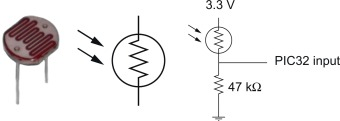 | Figure 2. Advanced Photonix PDV-P5002 [8] |
2.4. The Solar Cells (or Photovoltaic Cells)
- A solar cell produces an electric circuit when light falls on them. They are made of two layers of semiconductor materials like silicon. One is positively charged, while the other is negatively charged. When photons from light strike the solar cell, electrons are knocked loose from the atoms in the semiconductor material. The movement of electrons generates the DC electric current [10,11]. See figure 3.
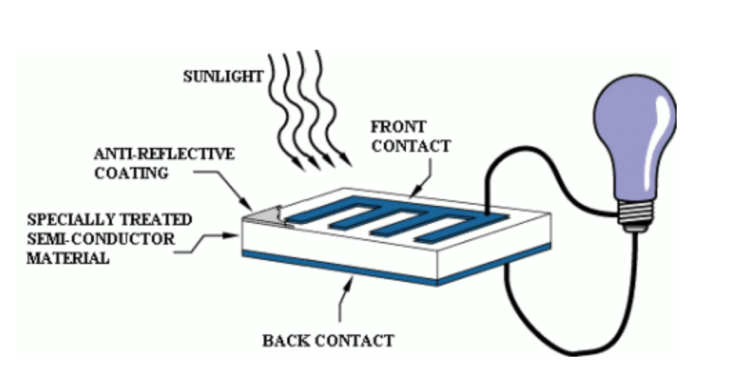 | Figure 3. Operations of a solar cell [11] |
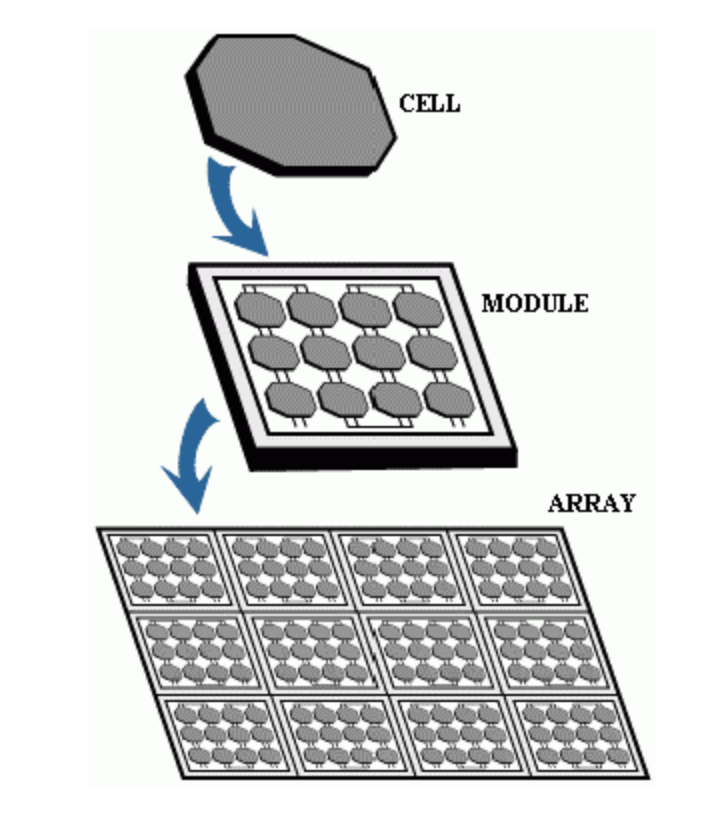 | Figure 4. Wiring multiple photovoltaic modules to form an array [11] |
 | Figure 5. Arrays of photovoltaic modules being installed on rooftops [15] |
 | Figure 6. Solar farm in Southern France [15] |
2.5. Solar Cells Systems Design
- Solar cells need to be mounted on a strong structure that can withstand severe weather. The structure should be angled to maximize the incident sunlight based on the local altitudes [18]. Batteries are used to store the generated electrical current for powering homes at night or on cloudy days. Since the PV cells generate DC current, inverters are used to convert it to AC current so it can flow through the electric grid and power homes [18].
2.6. Solar Cells Performance Factors
- Solar cells performance is the ratio of the electric power generated to the amount of incident light energy [19]. Solar cells perform optimally at lower temperatures since higher temperatures impact the properties of the semiconductor material increasing current but lowering voltage more. The performance of solar cells is impacted by extreme temperature increases as they can damage cells shortening operating lifetimes. Thermal management can reduce and avoid damage [20].Weather and environmental conditions like clouds, heat, pollution, dirt, and shade will impact and reduce the photovoltaic conversion efficiency. Solar backsheet, which is made from polymer and is placed on the solar panel, protects the cell from severe weather conditions and reduces its temperature [5]. New cells are being designed which will capture more light to increase electricity output (discussed in sec 2.9).A cell's efficiency can be increased by reducing the amount of reflected light from its surface. Untreated silicon reflects more than 30% of the incident light, which can be reduced by anti-reflection coatings and textured surfaces [20].The type of material from which solar cells are made impacts the efficiency. Solar cells made from monocrystalline pure silicon achieve an average efficiency of 18-20% while amorphous silicon achieves an average efficiency of 8-9%. Polycrystalline silicon achieves an efficiency of over 20% [17,20,23].
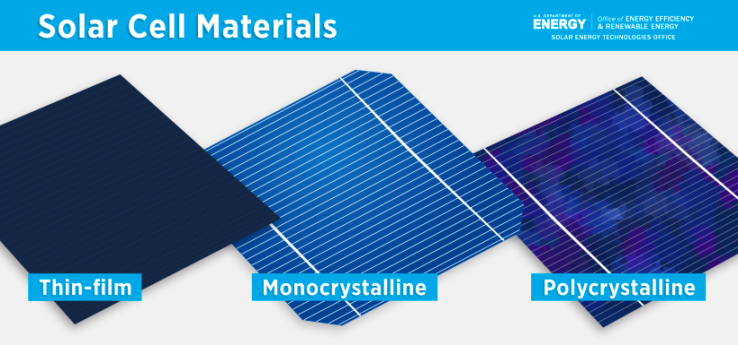 | Figure 7. Solar Cell Materials [23] |
2.7. Developments in Solar Photovoltaics
2.7.1. Perovskites Solar Cells
- Perovskites solar cells (see figure 8) have immense future renewable energy potential. Although initial developments occurred in 2006, they were published in 2009 and named in honor of Russian mineralogist Lev von Perovskite. Perovskite materials include methylammonium lead halides and have good light absorption properties due to their crystalline structure. Hence, the perovskite solar cells (PSCs) perform well under low and diffuse light [21,22].
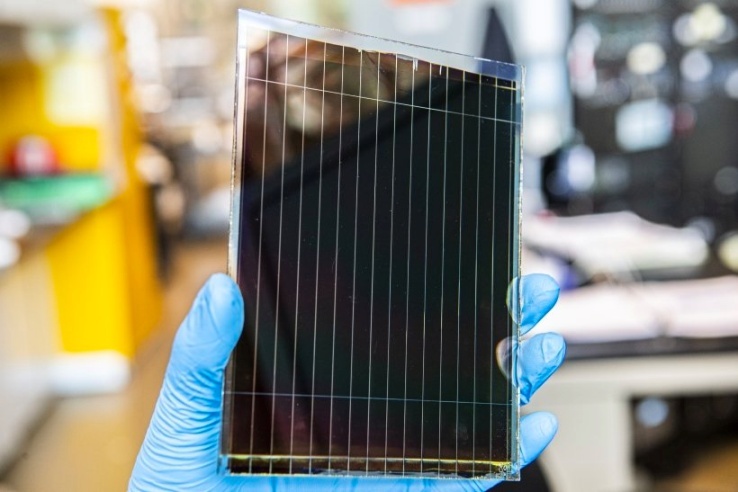 | Figure 8. Perovskite solar cells [9] |
2.7.2. Perovskites Solar Cells Challenges
- But there are four primary challenges that must be simultaneously addressed for perovskite technologies to be commercially successful. Power conversion efficiency is needed while achieving stability, durability, and scaling [24,25]. The stability of PSCs is vulnerable to extrinsic conditions like humidity, moisture, and elevated temperatures, and UV light. Intrinsic factors like defects in perovskite structure and ion migration can also impact the stability of PSCs. Validation, performance verification, and bankability are essential to the commercialization of perovskite technologies [24].
2.7.3. Crystalline Silicon Solar Cells
- These are the most common semiconducting material in solar panels. Its efficiencies range from 18-22% for commercially produced solar cells. It occupies more than 90% of the PV market in the world [22,26].
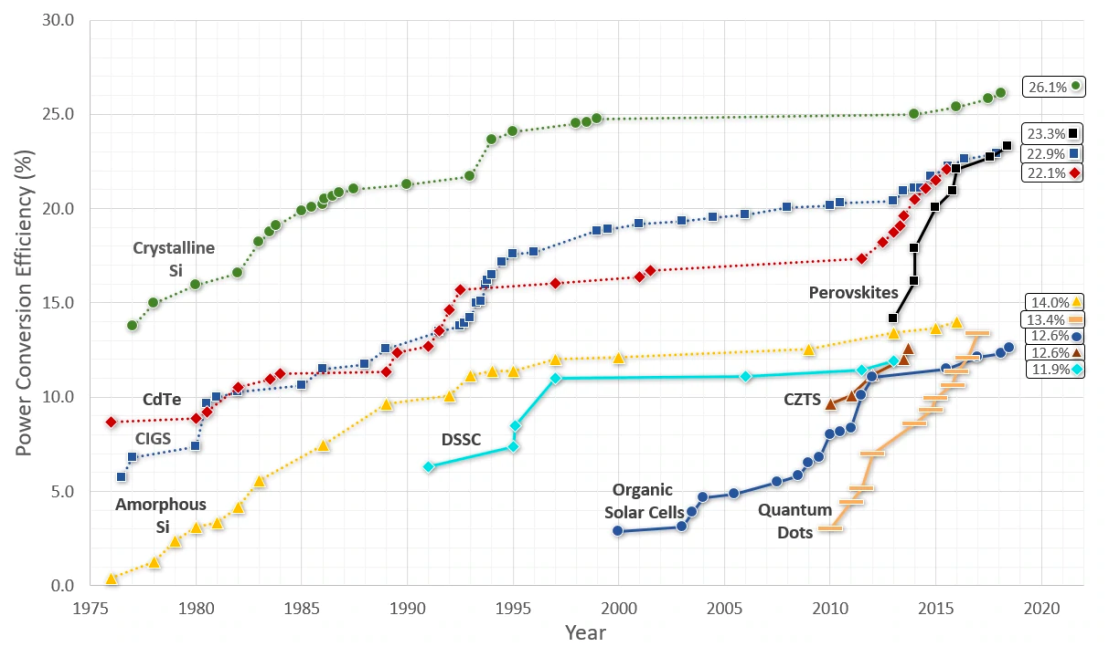 | Figure 9. Comparison of growth of Perovskite solar cells compared to other solar cell technologies [21] |
2.7.4. Concentrated Photovoltaic Cells (CPV)
- By converging light using lenses and mirrors over a small part of the solar cell, a large amount of current is generated. These cells have reached an efficiency f 38.9% [16].
2.7.5. Bifacial Solar Cells
- Si-based bifacial technology can produce electric current from both sides of the panel (front and back) when illuminate with light. Compared to standard monofacial panels, bifacial solar cells have 11% more efficiency. They have solar panels on both the front and rear sides. Figure 10 shows the Lumos Solar GSX bifacial modules [27].
 | Figure 10. Lumos Solar GSX bifacial modules [14] |
2.7.6. Thin-Film Solar Cells
- These are highly promising and said to be the future of the solar industry due to their lower cost, narrow design, ease of manufacturing, less waste, flexibility, and lower weight. They are mainly of 3 types. The amorphous silicon thin-film cells are easier to produce compared to crystalline cells. Cadmium-telluride (CdTe) thin-film cells are the second most popular after crystalline cells but need special handling due to cadmium's toxicity. In addition, tellurium is very rare to find. The copper-indium-gallium-selenide (CIGSe) thin-film cells are very promising due to their high efficiency of 21% and lower cost. Figure 11 shows an example of thin-film solar cells [22,28].
 | Figure 11. Thin-Film CIGSe solar cell [22] |
2.7.7. Quantum Dots Solar Cells
- Quantum dot (QD) solar cell technology is new and emerging and has the potential to increase the conversion efficiency to 66%. QDs are nanoscale (1-100 nm) semiconductor particles that act as key absorbing PV material. They can increase current generation efficiency since more than one bound electron-hole pair (or exciton) are excited per incoming photon versus other solar cells which generate only one bound electron-hole pair per photon [29,30].By tuning their bandgap, they can spread across wide energy levels making them suitable for multi-junction solar cells. They can emit light of various colors when illuminated by UV light. The wavelength of light emitted depends on its size. Longer wavelength light is emitted by QDs of larger size (5-6 nm) but shorter wavelength light is emitted by QDs of shorter size (2-3 nm). They have applications in solar cells, LEDs, quantum computing, microscopy, medical imaging, and fluorescent labels. Their properties are dependent on their size [31].
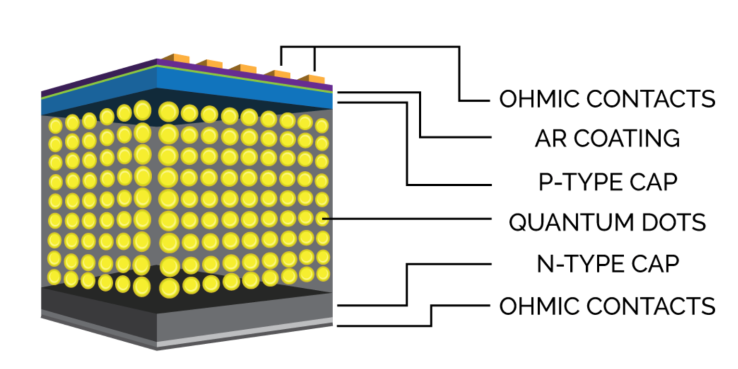 | Figure 12. Quantum dot solar cell [29] |
2.7.8. Organic Solar Cells (OSCs)
- OSCs are lightweight, made with carbon compounds, and have low fabrication costs. The electricity is conducted and generated with organic polymers and molecules [16].
2.7.9. Solar Cells Comparison
- Table 1 above shows the comparison between QD solar cells, OSCs, CPVs, and PSCs.
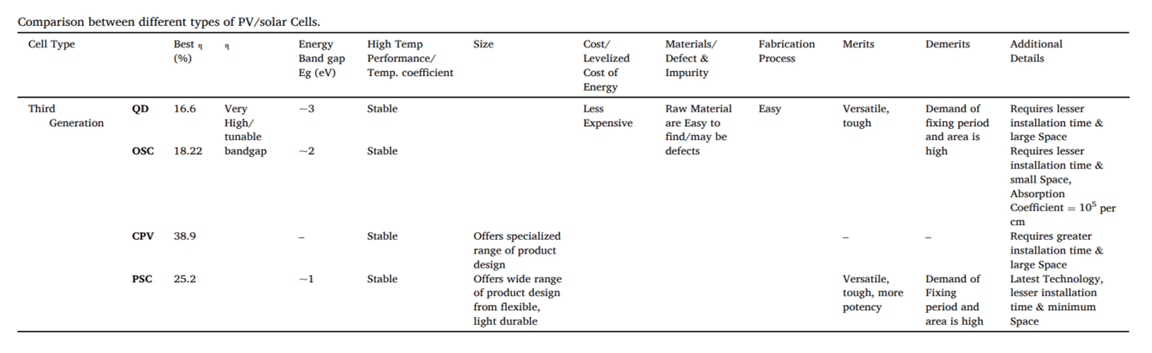 | Table 1. Solar cells comparison [16] |
3. Results
- Discovered by Heinrich Hertz in 1887 and explained by Albert Einstein in 1905 (for which he won the Nobel Prize in 1921), the photoelectric effect is significant because it demonstrates that light has particle-like qualities. It establishes that we can consider light as photons (packets) of energy where a photon interacts with an electron, and the photons must have sufficient energy to knock off each electron [1,2].The photoelectric effect has many applications. Since the electric current is triggered by light, it is widely used in light detection systems like "electric eye" door openers. Other applications of the photoelectric effect include photocopiers, photomultiplier tubes, photodiodes, phototransistors, scintillators, light meters used in photography, measuring the intensities of stars and their temperatures in astronomy, and producing electricity via solar cells [2,3,4,6,7].Its major application is in photovoltaic (PV) or solar cells. The PV cells are made of semiconductor material like silicon and produce power for an electrical circuit. Many solar cells are interconnected to form a solar module. Multiple modules are connected to form solar cell arrays or solar systems which are installed on rooftops to provide power to houses or buildings [11,10,5].Photovoltaic conversion efficiency is the module's ability to convert sunlight into electricity. High temperatures negatively impact a semiconductor's performance. Although current increases, it leads to a much larger decrease in voltage. If the material reflects light, it negatively impacts the cell's efficiency. Untreated silicon reflects more than 30% of the incident light, which can be reduced by anti-reflection coatings and textured surfaces. Dirt and shade also reduce the incident light negatively impacting the conversion efficiency [8,20]. With huge increases in conversion efficiency, from about 3% in 2006 to over 25% today, Perovskite solar cells have high performance and low production costs potential. However, they are facing some challenges before they can be fully commercialized including power conversion efficiency, stability and degradation, manufacturability, and technology validation. Other materials being developed which improve the conversion efficiency are crystalline silicon, bifacial solar cells, and thin-film solar cells [21,22].
4. Conclusions
- The photoelectric effect is one of Albert Einstein's greatest discoveries for which he won the Nobel Prize in Physics in 1921. It proves that light has particle-like characteristics in addition to its wave-like features since it contains many tiny packets of energy called "photons. This is also called the wave-particle duality of light [1,2]. The photoelectric effect, a key part of quantum mechanics, disproves the classical electromagnetism principle of light which says that light traveling as waves transfers energy to photoelectrons. It helps in drawing inferences on the properties of atoms, molecules, and solids [32]. Although the photoelectric effect is used in many applications, including astronomy, scintillators, and photocopiers, producing electricity from sunlight via solar cells is its main use in our daily lives [2,3,4,6,7]. Solar cells are wired to form modules, and many modules are combined to form solar system arrays. The arrays are installed on rooftops to produce electricity [5]. The conversion efficiency of photovoltaic cells is their ability to produce electricity from the incident sunlight. The conversion efficiency is negatively impacted by dirt, shade, and texture which reflects the sunlight [8,20].Technologies are being developed which can improve the performance of solar cells. Perovskite solar cells are the most promising based on high performance and low production costs, but some challenges need to be resolved prior to commercialization [22].
5. Future Research Direction
- The solar cells field is being actively researched. Many developments and improvements are being made in the Perovskite, crystalline silicon, bifacial, and thin-film solar cells technologies [22].Perovskite solar cells have a low cost and high-performance potential since their efficiency has trended up from 3% in 2006 to over 25% today. They have many potential applications in solutions processing and advanced manufacturing - light emitters, diodes, and low-power electronics. But some challenges on improving their stability and durability need to be addressed prior to commercialization [21,24].Crystalline silicon, bifacial solar cells, and thin-film solar cells are also being actively researched and developed. Bifacial solar cells went mainstream in 2010 but are currently used in large commercial installations. The challenge to it being adopted for residential installations is cost - it needs to be lowered [22,27].Multijunction solar cells are being actively researched. By stacking different semiconductor materials in layers, cell efficiency can be improved, but the cost of materials and fabrication needs to be lowered. Research is also being done to extend it to different PV cell technologies [23].Research is being carried out in organic solar cells to improve their lifetime and efficiency [16, 33]. The efficiency of quantum dot solar cell devices needs to be sufficiently increased for commercialization and research is being done in this area [31].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML