-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2018; 8(2): 35-45
doi:10.5923/j.ep.20180802.01

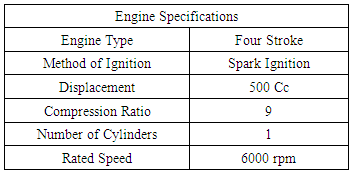
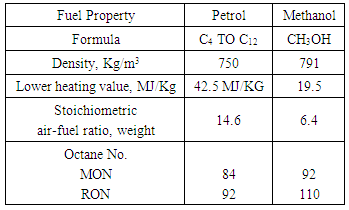
Thermodynamic Evaluation of Methanol as an Alternative Fuel to Gasoline in a Single Cylinder Spark Ignition Engine
M. Marouf Wani
Mechanical Engineering Department, National Institute of Technology, J&K, Srinagar, India
Correspondence to: M. Marouf Wani, Mechanical Engineering Department, National Institute of Technology, J&K, Srinagar, India.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper describes the results of the thermodynamic evaluation of methanol as a dedicated alternative fuel for gasoline based spark ignition engines. The investigations have been done for the octane demand of the engine for methanol under variable load and rated speed conditions. Further the difference in the octane ratings of the gasoline and methanol along with the investigated octane demand of the engine with both of these fuels is used for possible modifications needed to be done in the existing engine design to dedicate it to the methanol fuel only. The experimental engine modeled for thermodynamic analysis is a high speed 500cc single cylinder spark ignition engine used for motorcycle applications. Modeling and computational investigations for this engine was done in the professional thermodynamic internal combustion engine simulation software AVL BOOST. The design of the software is based on the conservation laws of mass, energy and momentum. The software uses numerical methods for solving the conservation laws including the 1-D Navier Stokes equations for generating the results for all thermodynamic and gas dynamic parameters in all engine components. The comparison was done for the thermodynamic output performance parameters like octane demand, power, torque and brake specific fuel consumption of the engine. The results were also generated for comparative CO, HC and NOx emissions characteristics of the engine. The thermodynamic results showing the lower octane demand of the engine for methanol implies that gasoline operated spark ignition engine needs changes in its design in order to be use with methanol fuel for better performance.
Keywords: Engine, Internal Combustion, Single-cylinder, Spark Ignition, Petrol, Methanol, Octane Requirement, Performance and Emissions
Cite this paper: M. Marouf Wani, Thermodynamic Evaluation of Methanol as an Alternative Fuel to Gasoline in a Single Cylinder Spark Ignition Engine, Energy and Power, Vol. 8 No. 2, 2018, pp. 35-45. doi: 10.5923/j.ep.20180802.01.
Article Outline
1. Introduction
- The design of conventional spark ignition and the compression ignition engine operated by gasoline and diesel fuels respectively has been subjected to many changes from time to time. One of the reasons is the developments in the electronic and computer technology fields. This development helped in the design of a better control system known as the engine management system. This technology nowadays is controlled by micro-controllers which ultimately helps in incorporating many other design and operating changes needed for improving the engine performance and emissions characteristics. The basic physical and the chemical properties of various internal combustion engine fuels are not same. Particularly in the field of spark ignition engines the properties of various fuels that are used in practice are deviated from each other. The fuels having advantage in terms of octane number rating, heating value, viscosity, density, freezing point, volumetric efficiency etc are regularly investigated for achieving better performance and emissions characteristics with future engine technology. The relative advantages present with the compression ignition engines like higher compression ratios, supercharging and turbo-charging are now adopted for investigations with spark ignition engine fuels as well on the spark ignition engines itself. The higher octane rating of the CNG has already made it possible to use it in basic diesel engine body having higher compression ratios and being ignited either by spark plug or by a pilot injection of diesel. Similarly the turbo-charged concept used for diesel based compression ignition engines is being tried with the high octane number rated spark ignition engine fuels in order to boost the power of such engines further, go for the downsizing of such engines. Further the most important need in the research investigations is to somehow bring down the pollutants like CO, HC and NOx from internal combustion engines. In addition to this the research is also being done to bring down the GHG emission levels from all automotive engines. Keeping all these current engine developments in mind it was decided to investigate the possibilities of better use of methanol in the existing commercial spark ignition engines. In order to achieve some positive results the investigations were started from the fundamental octane demand of the existing engine under constant rated speed of engine and variable load operation. It was expected that it will lead us to the modifications needed in the design and operating parameters of the spark ignition chosen for such research in order to improve its design for dedicated use of methanol fuel. Michael Saccullo et. al., conducted experimental investigations on a heavy duty single cylinder direct injection diesel engine to study the combustion efficiency and emissions for a range of load points. In order to combine the advantage of high fuel conversion efficiency of diesel engines and the advantage of lower particulate emissions with alcohol fuels, methanol or ethanol was used as the main fuel for this engine with a pilot injection of diesel to initiate combustion. Two standard common rail diesel injection systems were used on the same engine, one for main fuel alcohol and second for the pilot injection of diesel. The injection pressure for alcohol fuel was varied up to 1500 bar. The results were compared with its conventional heavy duty direct injection diesel engine version. The results were that the alcohol based dual fuel version resulted in comparable or even higher combustion efficiencies. Further the alcohol based dual fuel version produced lower particulates and NOx emissions. [1]Duc-Khanh Nguyen et. al., conducted studies on a direct injection spark ignition engine by using methanol in place of gasoline as an alternative fuel. They further used variable valve timing concept along with an intake boost control for the same. The results were the methanol fueled engine produced a brake mean effective pressure of 1.8 bar higher than the gasoline version at the speed of 1650rpm under WOT conditions. The BMEP improved further by 2.6 bar with positive valve overlap and higher intake boost pressure. At the BMEP of 16.3 bar the efficiency of the methanol version was higher by 22.7% with valve overlap control and by 25.75% with intake boost control. It was observed that the downsizing effect with boost control was higher than with variable valve timing. Further it was concluded that the engine in the methanol mode can be downsized by approximately 10.7% by boosting the intake pressure. [2]Pucilowski M., et. al., conducted experimental investigations on a heavy duty direct injection compression ignition engine using methanol as its fuel under a higher compression ratio of 27. The experiments were carried out by injecting the methanol with a common-rail injector at two injection pressures of 800 bar and 1600 bar. The results were that the NOx emissions were increased with higher injection pressure for methanol. Further numerical investigations were carried out to find the reason behind the increase in NOx emissions with increased injection pressure for methanol. The numerical simulations were carried by using Reynold Averaged Navier Stokes (RANS), Langrangian Particle Tracking (LPT) and Well-Stirred-Reactor (WSR) models. It was concluded that the higher injection pressure changed the fuel vapor penetration length resulting in change in ignition delay time. This effected the high temperature zone of the engine cylinder with an increase in the rate of NOx emissions produced by the engine. [3]Zhanming Chen et. al., conducted experimental investigations on a turbo-charged spark-ignition engine fuelled with natural gas and methanol to investigate the combustion characteristics such as in-cylinder pressure, heat release rate, burned mass fraction, knock intensity, ignition delay, centroid of heat release rate, and coefficient of variation of indicated mean effective pressure under light loads using 0%, 16%, 34% and 46% methanol substitution rates. The results were that the combustion phase advanced with the increase in methanol substitution rate due to faster burning velocity of methanol. Knock occurred with methanol substitution rate of 46% at 2000rpm. When the methanol substitution rate rose from 0% to 46%, the centroid of heat heat releaserate shifted from 7.23 ATDC to 5.52 degree ATDC. The maximum in cylinder pressure rose from 46.1 bar to 53.5 bar and the crank angle corresponding to maximum in cylinder pressure similar with combustion phenomenon at 1200rpm, the combustion phase advance by and large at 2000rpm as well. Moreover shorter ignition delay, higher in-cylinder pressure and large rate of pressure rise were observed with methanol as compared to gasoline mode. [4]Leonid Tartakovsky et. al., conducted experiments, with gasoline, on a single cylinder spark ignition engine fitted with a carburetor and used as gen-set. The engine was next converted into its direct-injection version and experiments were repeated with gaseous hydrogen-rich methanol reforming products. The results showed that the methanol steam reforming products had a great potential for reducing the pollutants as compared with gasoline. The particulate emissions were mitigated to zero impact level. The efficiency of the methanol reformate direct injection engine was 20-70% higher than that of the carburetor-fed gasoline engine. [5]Erik Svensson et. al., conducted investigations on the emission potential of methanol and diesel using 0-D reactor based T-¢ maps, stochastic reactor model (SRM) based engine simulations and finally the experimental verification on a truck engine running in partially premixed combustion at medium loads. The T-¢ maps used constant pressure, constant temperature and constant equivalent ratio lines. It was found that the experiments validated the CO and NOx emissions. However the HC emissions were underestimated by the computational methods. Finally the trajectories of SRM simulations were superimposed on the T-¢ maps. It was found from the T- ¢ maps that the soot emissions were non-existent. In general the emissions with methanol fuel were lower than those from diesel. However the CO and NOx levels for methanol and diesel fuels were same. The SRM simulations as well as the engine based experiments confirmed these findings. [6]Fan Zhang et. al., conducted experimental investigations on a gasoline engine fitted with PFI system and using Euro III gasoline, M10, M15, M20 and M30 as fuels. The results were that methanol gasoline blend fuel could adapt to current engines and vehicles. Further the fuel consumption, THC and CO emissions were less with methanol gasoline blends as compared to pure gasoline. [7]Sukho Jung, et. al., conducted experimental investigations on a single cylinder diesel engine using methanol and natural gas as two alternative fuels and DME as an ignition source. They studied the effect of fuel cetane number or low temperature reaction (LTR), high temperature reaction (HTR), knock limit temperature and misfire limit between methanol and natural gas. The results showed that the ignition temperatures of LTR and HTR is dependent only on cetane number of the fuel. Also the maximum heat release rate of LTR is dependent not only on cetane number but also the fuel composition. [8]Ramadan B et al., conducted numerical investigations with methanol on a direct injection 4 stroke cycle spark ignition engine using two types of bowl-in-pistons, under swirl and no-swirl conditions and under variable air-fuel ratios. The investigations were done for the closed period of the cycle only. The results showed that the fuel-air mixing, combustion and flame propagation were significantly improved when swirl was turned on. This further resulted higher peak pressures in the cylinder as well as heat loss across the cylinder walls. The investigations further showed incomplete combustion under stoichiometric operation of the engine. [9]Brusstar M et al., conducted experimental investigations on a medium duty turbocharged spark ignition engine, using ethanol and methanol blends with petrol with port fuel injection system and high compression ratios. The alcohols used were derived from the renewable biomass source. The results indicated that the bio-derived fuels were cost effective and resulted in efficient operation of the engine. [10]Zhang Fan et al., conducted computational and experimental investigations on a SI engine using M10, M20 and M30 as methanol-gasoline blended fuels for estimating aldehyde emissions formation. The computation was done using AVL BOOST software and the experimental investigations were conducted using Fourier transform infrared spectrometer. Both the simulated as well as experimental results show that the formaldehyde emissions increased with increased percentage of methanol in the methanol-gasoline blends. [11]Methanol can be produced from natural gas, coal and wood. Some favorable physical and chemical properties of a mixture of 85 percent methanol and 15 percent gasoline known as M85 make it possible to use it as an alternative liquid fuel for spark ignition engine based vehicles. However difficulties with methanol arise from its low energy, a non-visible flame with M100 (neat methanol), cold starting difficulties, lubricant contamination, increased engine wear, increased formaldehyde emissions, materials incompatibility. [12]Fleming R. et al., conducted experimental investigations with methanol on a single-cylinder research engine, a 4-cylinder 122-CID (2000 cc) engine, and a 8-cylinder 350-CID engine. The results showed that the single cylinder engine could operate leaner than the multi-cylinder engine since non-uniform distribution of air-fuel mixture in multi-cylinder engine for each cycle was observed. The methanol based engines were 5% more economical than their corresponding gasoline versions. Also the methanol based engines produced substantially lower nitrogen oxides emissions. [13]Harrington J., et al. conducted experimental investigations on a single cylinder engine with methanol and indolene as two fuels over wide range of speed and load. The results showed that methanol fuel exhibits faster burning rate, (shorter ignition delay period and combustion interval). Also with same engine air flows and equivalence ratios, methanol produced more power than indolene. Methanol based engine consumed more fuel but energy consumption rate was lower with methanol. The methanol engine produced lower NO emissions but CO and HC emissions were higher in any one of the two versions depending upon the operating value of the equivalence ratio. [14]Sumio Ito et al., conducted fundamental research on a conventional spark ignition engine using methanol as its fuel. The results showed that the thermal efficiency was greater with methanol. Also methanol showed pre-ignition problems by increasing the compression ratio of the engine. The unburned fuel and aldehyde emissions were greater with the methanol fuel but the evaporative emissions were lower with methanol. The methanol showed cold start problems which was overcome in the dual fuel mode. [15]Gardiner D et al., conducted experimental investigations on a small spark ignition engine under sub-zero temperature conditions to study the comparative cold start problems with methanol, indolene and commercial gasoline fuels. The results showed that the methanol based engine failed to start at below about zero degree Celsius temperature whereas the indolene fuelled engine could start easily up to - 45 degree Celsius temperatures. [16]Adelman H conducted experimental investigations with methanol in both spark ignition combustion and compression ignition combustion types of engine designs. The compression ignition combustion engine design was modified by incorporating an additional spark ignition system in it to be used for both diesel and methanol operation. The results showed that, in case of the compression ignition combustion based engine design, the methanol fuel eliminated the particulates and also produced lesser NOx emissions. However the fuel consumption was higher with methanol as compared to diesel as its fuel. Again with methanol as fuel, the modified compression ignition combustion type of engine design gave higher thermal efficiency than the conventional spark ignition type of engine design. The NOx and CO emissions were comparable with both the types of designs mentioned above. Also the unburned fuel emissions were higher with the modified compression ignition type of engine design. [17]Pannone G et al., conducted experimental investigations with methanol on a single cylinder spark ignition engine under naturally aspirated and turbocharged conditions under varying air- fuel ratios. The results were that lean operation of turbocharged engine gave higher thermal efficiency as compared to stoichiometric operation of naturally aspirated version. Also the carbon monoxide and nitrogen emissions were reduced in case of the turbocharged engine. However the unburned fuel and the aldehyde emissions were increased with the turbocharged version. [18]Niwa K et al., conducted controlled fleet tests with M85 fuel on Otto-type vehicles in Japan. The results confirmed it as a fuel for Otto-type vehicles in Japan with improved and durable fuel injector technology, improved cold start ability design and reduced formaldehyde emissions technology. [19]The conclusions taken from the literature suggest that methanol is a suitable fuel for spark ignition engines. Further there are possibilities to improve the overall performance of spark ignition engines with the possible dedicated methanol engine.
2. Theoretical Basis [20]
2.1. The Cylinder, High Pressure Cycle, Basic Equation
- The calculation of the high pressure cycle of an internal combustion engine is based on the first law of thermodynamics:
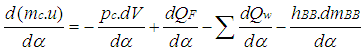 | (1) |
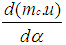 = change of the internal energy in the cylinder.
= change of the internal energy in the cylinder. 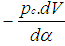 = piston work.
= piston work. 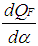 = fuel heat input.
= fuel heat input. 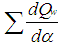 = wall heat losses
= wall heat losses 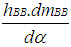 = enthalpy flow due to blow-by
= enthalpy flow due to blow-by 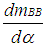 = blow-by mass flowThe first law of thermodynamics for high pressure cycle states that the change of internal energy in the cylinder is equal to the sum of piston work, fuel heat input, wall heat losses and the enthalpy flow due to blow-by.In order to solve this equation, models for the combustion process and the wall heat transfer, as well as the gas properties as a function of pressure, temperature, and gas composition are required.Together with the gas equation
= blow-by mass flowThe first law of thermodynamics for high pressure cycle states that the change of internal energy in the cylinder is equal to the sum of piston work, fuel heat input, wall heat losses and the enthalpy flow due to blow-by.In order to solve this equation, models for the combustion process and the wall heat transfer, as well as the gas properties as a function of pressure, temperature, and gas composition are required.Together with the gas equation 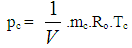 | (2) |
2.2. Combustion Model
- Air Requirement and Heating Value modeling is given below.
2.2.1. Stoichiometric Air-Fuel Mixture
- The following equation for the stoichiometric air requirement specifies how much air is required for a complete combustion of 1 kg fuel:
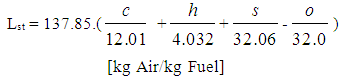 | (3) |
2.2.2. Lean Mixture
- For lean combustion, the total heat supplied during the cycle can be calculated from the amount of fuel in the cylinder and the lower heating value of the fuel.
2.2.3. Rich Mixture
- In rich air fuel mixture combustion, the total heat supplied during the cycle is limited by the amount of air in the cylinder. The fuel is totally converted to combustion products even if the amount of air available is less than the amount of stoichiometric air.
2.2.4. Heating Value
- The lower heating value is a fuel property and can be calculated from the following formula:
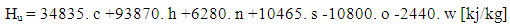 | (4) |
2.2.5. Heat Release Approach
- Vibe Two ZoneThe rate of heat release and mass fraction burned is specified by the Vibe function given by equation No.5 below.The first law of thermodynamics is applied separately to the burned and unburned mixture while assuming that the temperatures of these two mixtures is different.
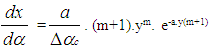 | (5) |
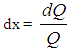 | (6) |
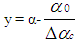 | (7) |
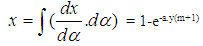 | (8) |
2.3. Gas Exchange Process
- Basic EquationThe equation for the simulation of the gas exchange process is also the first law of thermodynamics:
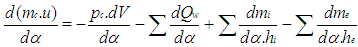 | (9) |
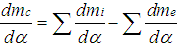 | (10) |
2.4. Piston Motion
- Piston motion applies to both the high pressure cycle and the gas exchange process.For a standard crank train the piston motion as a function of the crank angle α can be written as:
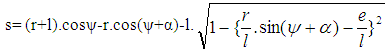 | (11) |
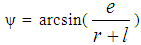 | (12) |
2.5. Heat Transfer
- The heat transfer to the walls of the combustion chamber, i.e. the cylinder head, the piston, and the cylinder liner, is calculated from:
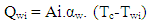 | (13) |
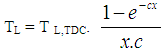 | (14) |
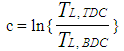 | (15) |
2.5.1. Woschni Model
- The woschni model published in 1978 for the high pressure cycle is summarized as follows:
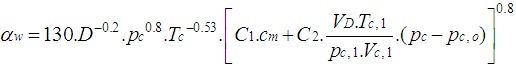 | (16) |
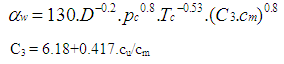 | (17) |
2.6. Fuel Injector
- The fuel injector model is based on the calculation algorithm of the flow restriction. This means that the air flow rate in the fuel injector depends on the pressure difference across the injector and is calculated using the specified flow coefficients. For the injector model, a measuring point must be specified at the location of the air flow meter. In this case the mean air flow at the air flow meter location during the last complete cycle is used to determine the amount of fuel. As is the case for continuous fuel injection, the fuelling rate is constant over crank angle.
2.7. Pipe Flow
- The one dimensional gas dynamics in a pipe are described by the continuity equation
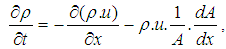 | (18) |
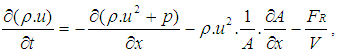 | (19) |
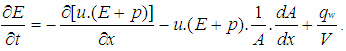 | (20) |
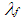 :
: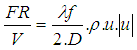 | (21) |
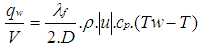 | (22) |
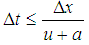 | (23) |
2.8. Knock Model
- Ignition Delay and Octane Number RequirementAVL Boost uses the following equation based model proposed by Hires etal. for the calculation of ignition delay in combustion.
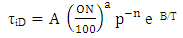 | (24) |
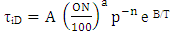 τiD = ignition delayON = Octane Number RequirementA = 17.68 msB = 3800 Ka = 3.402n = 1.7
τiD = ignition delayON = Octane Number RequirementA = 17.68 msB = 3800 Ka = 3.402n = 1.73. Methodology Used in Present Investigations
- 1. First of all a thermodynamic model for the single cylinder spark ignition engine was created in AVL BOOST software using various suitable models.2. Various suitable models for octane demand, heat transfer, combustion, frictional power etc. were selected for the above model.3. The values for the design and operating parameters of the engine were given as input to the model.4. Computations were carried out under constant rated speed and variable load operation on full cycle simulation basis.5. The results were generated for the octane demand of engine, power, torque, BSFC and the CO, HC and NOx emissions produced by the modeled engine.6. The above procedure was repeated twice by selecting one fuel with the corresponding input values for the modeled engine as well as the operating parameters.7. The comparison of the results was done to find out possibilities for improving the design and performance of the experimental engine with methanol fuel.
4. Results and Discussions
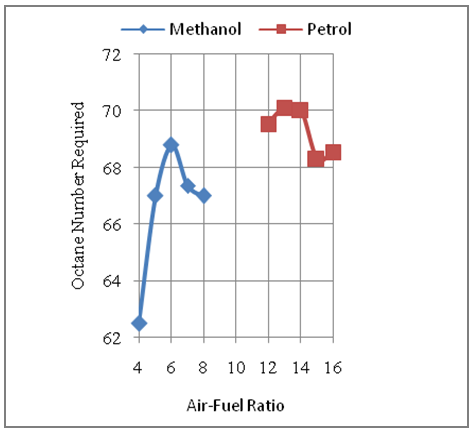
4.1. Effect of Air-Fuel Ratio on Octane Number Requirement
- The Fig.1 below shows the octane number requirement for methanol and gasoline fuels under constant speed of 6000rpm and variable air-fuel ratio. The air-fuel ratio ranges for the two fuels methanol and gasoline are from extreme rich mixture to extreme lean mixture conditions as per the stoichiometric air-fuel ratios for each of these two fuels.
 | Figure 1. Effect of Air-Fuel Ratio on Octane Demand of the Engine |
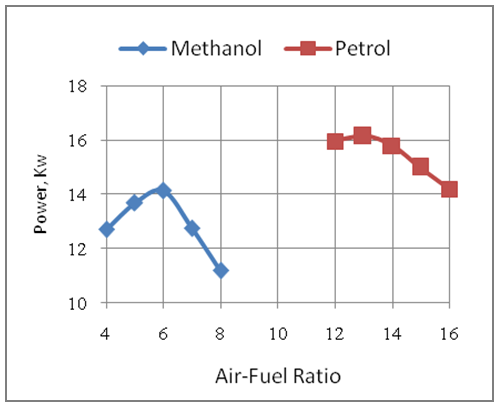
4.2. Effect of Air-Fuel Ratio on Engine Power
- The Fig.2 below shows the effect of air-fuel ratio on the power developed by the engine with methanol and gasoline as its fuels. It is seen that the engine develops higher power with gasoline fuel as compared to methanol fuel. This is because of more amount of heat being released during combustion with gasoline as compared to methanol under same design and similar operating conditions. The higher pressures and temperatures developed with gasoline develop more power than that developed in the methanol mode. This is due to the combined effect of the heating values and stoichiometric air-fuel ratios of the two fuels for the same displacement volume of the engine. The maximum power produced with gasoline is 16.15Kw as compared to 14.12Kw produced with methanol fuel under same design and similar operating conditions. The engine produces more power under rich operation. The power developed by the engine comes down in lean modes for both gasoline and methanol fuels as the torque developed comes down due to less amount of heat being released under these conditions.
 | Figure 2. Effect of Air-Fuel Ratio on Power Developed by the Engine |
4.3. Effect of Air-Fuel Ratio on Engine Torque
- The Fig.3 below shows the effect of air-fuel ratio on the torque developed by the engine with two fuels methanol and gasoline. It is clear that the torque developed with gasoline is higher than that developed with methanol. The engine develops higher torque with gasoline than methanol because of higher pressures being developed with gasoline fuel. This is because of favorable effects of heating values and combustion stoichiometry with gasoline for the same displacement volume under consideration. The maximum torque developed with methanol fuel is 22.43Nm as compared to 25.68Nm produced with gasoline fuel. The torque produced by the engine with gasoline fuel is highest at an air-fuel ratio of 13 and goes down towards further rich as well as leaner conditions. This is because the combustion characteristics with gasoline are best at an air-fuel ratio of 13. The engine produces the highest torque at an air-fuel ratio of 6 with methanol. The torque developed goes down with further leaner as well as richer mixtures. This is because with methanol as a fuel the combustion efficiency is highest at an air-fuel ratio of 6.
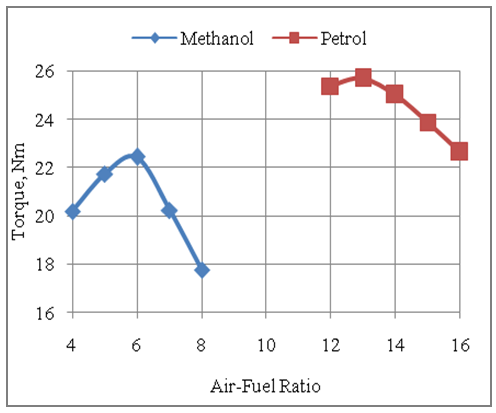 | Figure 3. Effect of Air-Fuel Ratio on Torque Developed by the Engine |
4.4. Effect of Air-Fuel Ratio on Brake Specific Fuel Consumption
- The Fig.4 below shows the effect of air-fuel ratio on the brake specific fuel consumption of the engine with methanol and gasoline fuels. It is seen from the graph that the fuel consumption per unit of energy output with methanol fuel is higher than that with gasoline. The maximum and minimum fuel consumption per unit of energy output with methanol are 1212.5grams and 738 grams respectively as compared to the maximum and minimum fuel consumption per unit of energy output of 382.5 grams and 325grams respectively for gasoline fuel. Also the engine consumes less fuel under lean operation, with either of the two fuels under consideration, because more availability of the air or oxygen under lean conditions makes it possible to consume entire amount of fuel during combustion.
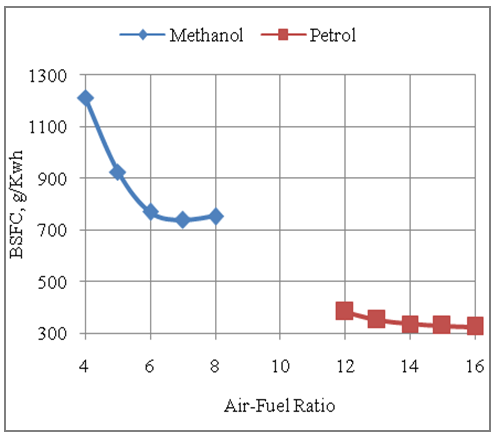 | Figure 4. Effect of Air-Fuel Ratio on Brake Specific Fuel Consumption |
4.5. Effect of Air-Fuel Ratio on CO Emissions
- The Fig.5 below shows the effect of air-fuel ratio on the CO emissions produced by the engine with methanol and gasoline as its fuels. It is seen that the engine produces more CO emissions with methanol fuel as compared to gasoline fuel per unit of energy output. The CO emissions per unit of energy output are higher with methanol due to higher mass of methanol consumption per unit of energy output as compared to gasoline fuel. The fuel consumption with methanol is at least double than the fuel consumption with gasoline under same design and similar operating conditions. Further the engine produces less CO emissions under leaner operation, with either of the fuels under consideration, as compared to rich operation due to availability of more air or oxygen for better combustion. The maximum CO emissions produced with methanol is 600g/Kwh as compared to 312.6g/Kwh of CO emissions produced with gasoline fuel. The CO emissions produced by the engine with either of the two fuels touches the zero line at the upper end of their lean operation limits respectively.
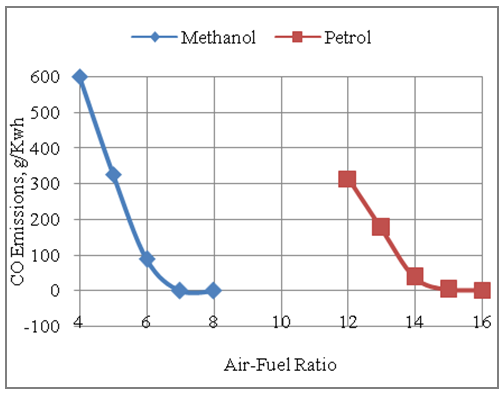 | Figure 5. Effect of Air-Fuel Ratio on CO Emissions |
4.6. Effect of Air-Fuel Ratio on HC Emissions
- The Fig.6 below shows the effect of air-fuel ratio on HC emissions with methanol and gasoline fuels. It is seen that the engine produces more HC emissions per unit of energy output with methanol as compared to gasoline as the methanol consumption per unit of energy output is higher than that of gasoline. Further it is seen that the HC emissions produced by the engine are reduced under leaner operation due to better combustion with excess air under leaner operation. It is seen that the maximum HC emissions per unit of energy output produced with gasoline fuel under rich operation is 1.77g/Kwh as compared to 5.0g/Kwh of HC emissions produced with the corresponding rich operation of the engine with methanol fuel.
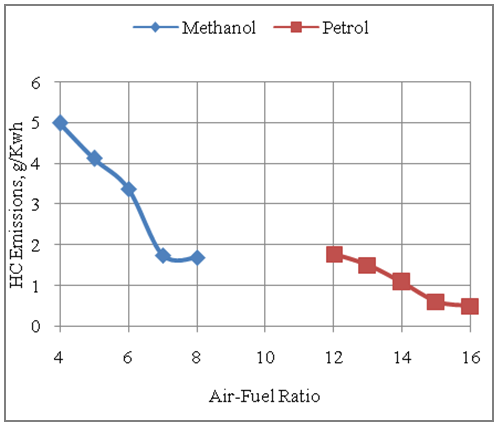 | Figure 6. Effect of Air-Fuel Ratio on Hydrocarbon Emissions |
4.7. Effect of Air-Fuel Ratio on NOx Emissions
- The Fig.7 below shows the effect of air-fuel ratio on the formation of NOx emissions with methanol and gasoline as two alternative fuels. It is seen from tha graph that gasoline based engine produces more NOx emissions than methanol fuelled engine. The gasoline version produces more emissions tan the methanol based engine as higher temperatures are produced in the gasoline engine as compared to the methanol fuelled engine. This is due to better combustion with gasoline fuel as compared to methanol fuel under same design and similar operating conditions. The higher temperatures produced in case of gasoline fuel helps to convert more mass of nitrogen of air in the engine cylinder into its oxides. The peak NOx emissions are produced with slightly lean mixtures with both the fuels under consideration. This is because the factors like availability of oxygen and nitrogen of air in the combustion chamber and the development of high temperatures during combustion do exist under slightly leaner operation of the engine in the two modes. The maximum NOx emissions per unit of energy output produced with gasoline fuel is 11.87g/Kwh as compared to 2.12g/Kwh of NOx produced per unit of energy output with methanol fuel under same design and similar operating conditions respectively.
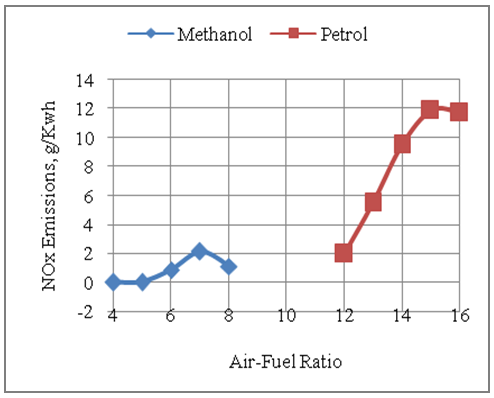 | Figure 7. Effect of Air-Fuel Ratio on NOx Emissions |
4.8. Effect of Air-Fuel Ratio on Exhaust Gas Temperature
- The Fig.8 below shows the effect of air-fuel ratio on the exhaust gas temperature produced with gasoline and methanol fuels. It is seen that higher temperatures are produced with gasoline fuel as compared to methanol fuel because of higher heating value of gasoline as compared to methanol. This together with better combustion with gasoline fuel under same design and similar operating conditions of the engine results in the development of higher exhaust gas temperatures with gasoline fuel as compared to methanol.
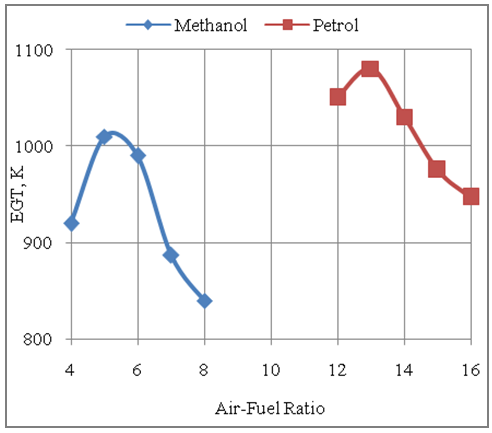 | Figure 8. Effect of Air-Fuel Ratio on Exhaust Gas Temperature |
5. Conclusions
- 1. Methanol can successfully be used as a fuel in spark ignition engines.2. As per the results on octane demand of the engine for methanol it is concluded that higher compression ratios can be used for such engines when operated with methanol fuel.3. Also low pressure ratio based turbo-chargers can also be tried for power boosting of such engines.4. Use of higher compression ratio or low level boost will help in reducing the gap between the performances of this engine under gasoline and methanol modes.5. Finally it is concluded that there is a good potential for using huge sources of methanol as a fuel for spark ignition engines as and when the demand is shifted towards it.
ACKNOWLEDGEMENTS
- Author is thankful to AVL Austria and its unit AVL India Ltd Gurgaon for providing BOOST engine simulation software with free license for academic research purposes.
Appendix-A
- Nomenclaturea = speed of soundA = pipe cross-sectionAeff = effective flow areaAi = surface area (cylinder head, piston, liner)AFCP = air fuel ratio of combustion productsAgeo = geometrical flow areac = mass fraction of carbon in the fuelcV = specific heat at constant volumecp = specific heat at constant pressureC1 = 2.28+0.308.cu/cmC2 = 0.00324 for DI enginesCm = mean piston speedCu = circumferential velocitycu = circumferential velocityD = cylinder boreD = pipe diameterdmi = mass element flowing into the cylinderdme = mass element flowing out of the cylinderdvi = inner valve seat diameter (reference diameter)
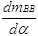 = blow-by mass flowe = piston pin offsetE = energy content of the gas
= blow-by mass flowe = piston pin offsetE = energy content of the gas 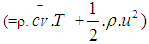 f = fraction of evaporation heat from the cylinder chargeFR = wall friction forceh = mass fraction of hydrogen in the fuelhBB = enthalpy of blow-byhi = enthalpy of in-flowing masshe = enthalpy of the mass leaving the cylinderHu = lower heating valuek = ratio of specific heatsl = con-rod lengthm = shape factor
f = fraction of evaporation heat from the cylinder chargeFR = wall friction forceh = mass fraction of hydrogen in the fuelhBB = enthalpy of blow-byhi = enthalpy of in-flowing masshe = enthalpy of the mass leaving the cylinderHu = lower heating valuek = ratio of specific heatsl = con-rod lengthm = shape factor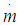 = mass flow ratemc = mass in the cylinder mev = evaporating fuelmpl = mass in the plenumn = mass fraction of nitrogen in the fuelo = mass fraction of oxygen in the fuelp = static pressureP01 = upstream stagnation pressurePc,o = cylinder pressure of the motored engine[bar]Pc,1 = pressure in the cylinder at IVC[bar]ppl = pressure in the plenumpc = cylinder pressurep2 = downstream static pressureqev = evaporation heat of the fuelqw = wall heat flowQ = total fuel heat inputQF = fuel energyQwi= wall heat flow (cylinder head, piston, liner)r = crank radiusR0 = gas constants = piston distance from TDCt = time T = temperatureTc,1 = temperature in the cylinder at intake valve closing (IVC)Tc = gas temperature in the cylinderTwi = wall temperature (cylinder head, piston, liner)TL = liner temperatureT L,TDC = liner temperature at TDC positionT L,BDC = liner temperature at BDC positionTw = pipe wall temperatureT01 = upstream stagnation temperatureu = specific internal energyu = flow velocityV = cylinder volumeV = cell volume (A.dx)VD = displacement per cylinderw = mass fraction of water in the fuelx = relative stroke (actual piston position related to full stroke)x = coordinate along the pipe axisα = crank angleαo = start of combustionΔαc = combustion durationαw = heat transfer coefficientρ = densityμσ = flow coefficient of the portψ = crank angle between vertical crank position and piston TDC position
= mass flow ratemc = mass in the cylinder mev = evaporating fuelmpl = mass in the plenumn = mass fraction of nitrogen in the fuelo = mass fraction of oxygen in the fuelp = static pressureP01 = upstream stagnation pressurePc,o = cylinder pressure of the motored engine[bar]Pc,1 = pressure in the cylinder at IVC[bar]ppl = pressure in the plenumpc = cylinder pressurep2 = downstream static pressureqev = evaporation heat of the fuelqw = wall heat flowQ = total fuel heat inputQF = fuel energyQwi= wall heat flow (cylinder head, piston, liner)r = crank radiusR0 = gas constants = piston distance from TDCt = time T = temperatureTc,1 = temperature in the cylinder at intake valve closing (IVC)Tc = gas temperature in the cylinderTwi = wall temperature (cylinder head, piston, liner)TL = liner temperatureT L,TDC = liner temperature at TDC positionT L,BDC = liner temperature at BDC positionTw = pipe wall temperatureT01 = upstream stagnation temperatureu = specific internal energyu = flow velocityV = cylinder volumeV = cell volume (A.dx)VD = displacement per cylinderw = mass fraction of water in the fuelx = relative stroke (actual piston position related to full stroke)x = coordinate along the pipe axisα = crank angleαo = start of combustionΔαc = combustion durationαw = heat transfer coefficientρ = densityμσ = flow coefficient of the portψ = crank angle between vertical crank position and piston TDC position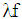 = wall friction coefficientΔt = time stepΔx = cell length
= wall friction coefficientΔt = time stepΔx = cell length
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML