-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2017; 7(3): 65-69
doi:10.5923/j.ep.20170703.02

Comparative Study of Waste Cooking Oil and Cashew Nut Shell Oil Bio Fuel Blends with Diesel
G. Sujaykumar1, Ravikumar R.2, Sushilendra Mutalikdesai1, Basavaraj Pujar1, Vinayak Baddi1, Dhareppa Siddapur1, Santosha P. V.1, Basavaraj Telagadi1
1Department of Mechanical Engineering, Yenepoya Institute of Technology, Moodbidri, India
2Department of Mechanical Engineering, Faculty of Engineering, Christ University, Bengaluru, India
Correspondence to: G. Sujaykumar, Department of Mechanical Engineering, Yenepoya Institute of Technology, Moodbidri, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Palm Waste Cooking Oil (WCO) is harmful waste product from food industries and restaurants. WCO can be used as bio diesel by some processes and additives. In other hand, Cashew Nut Shell Oil (CNSO) is produced from waste shell collected from Cashew Nut Industry by additives and processes. Both bio fuels produced are blended with diesel with same percentage of 10%, 20%, 30% and 40%. Laboratory tests and analyses are carried to study properties like fire point, viscosity and calorific value of blends of both the fuels. Both Dynamic and Kinematic viscosity are high in case of CNSO and there is small change in the viscosity with increase in the temperature. But WCO blends viscosity decreases with higher value compare to CNSO blends. Calorific value is lesser for WCO blends compare to CNSO blends. Observations shows, CNSO is more reactive than WCO. CNSO blends can retain their viscosity in high temperature so it will provide lubrication.
Keywords: Cold press method, Viscosity, Calorific value
Cite this paper: G. Sujaykumar, Ravikumar R., Sushilendra Mutalikdesai, Basavaraj Pujar, Vinayak Baddi, Dhareppa Siddapur, Santosha P. V., Basavaraj Telagadi, Comparative Study of Waste Cooking Oil and Cashew Nut Shell Oil Bio Fuel Blends with Diesel, Energy and Power, Vol. 7 No. 3, 2017, pp. 65-69. doi: 10.5923/j.ep.20170703.02.
Article Outline
1. Introduction
- The current methods to produce, convert and consume energy derived from fossil fuels throughout the world are not sustainable. Due to depletion of fossil fuels and increasing the concerns of global warming, there is an ever growing urge to develop fuel substitutes that are renewable and sustainable. Biodiesel is well-accepted alternatives to diesel fuel as they are economically feasible, renewable, environmental-friendly and can be produced easily in rural areas where there is an acute need for modern forms of energy. Edible vegetable oils such as canola, soybean, and corn have been used for biodiesel production and are proven diesel substitutes [1, 2]. However, a major obstacle in the commercialization of biodiesel production from edible vegetable oils is their high production cost, which is due to the demand for human consumption. Reducing the cost of the feedstock is necessary for biodiesel’s long- term commercial viability. One way to reduce the cost of this fuel is to use less expensive feedstocks including waste cooking oils and vegetable oils that are non-edible and/or require low harvesting costs. Waste cooking oil (WCO), which is much less expensive than edible vegetable oil, is a promising alternative to edible vegetable oil. Waste cooking oil and fats set forth significant disposal problems in many parts of the world. This environmentally-threatening problem could be turned into both economic and environmental benefit by proper utilization and management of waste cooking oil as a fuel substitute [3, 4]. Cashew Nut Shell Liquid (CNSO) is a reddish brown viscous liquid, having the honey comb structure of the shell of cashew nut obtained from cashew tree. CNSO is a versatile by-product of the cashew industry. The nut has a shell of about 1/ 8 inch thickness inside which is a soft honey comb structure containing a dark, reddish brown viscous liquid. It is called cashew nut shell liquid, which is the fluid of the cashew nut. It is often considered as the better and cheaper, material for unsaturated phenols [5, 6]. CNSO has innumerable applications in polymer based industries such as friction linings, paints and varnishes, laminating resins, rubber compounding, resins, cashew cements, polyurethane, based polymers, surfactants, epoxy, resins, foundry chemicals and, intermediates for chemical industry. It offers much scope and varied opportunities for the development of other tailor - made polymers [7, 8]. Studies on extraction and isolation of cashew nut liquid are done and found various methods for extraction of cashew nut shell oil like roasting method, hot oil bath method, screw press method, solvent extraction, and extraction using supercritical carbon dioxide [9, 10]. Work can be focused on using different solvents and combination of solvents for extraction of CNSO from Indian cashew nut shell, both for Steam roasted shells as well raw cashew nut shells and their yields at different solute to solvent ratios. This enables optimum solute to solvent ratios for extraction of CNSO [11, 12].
2. Methodology
2.1. Production of Waste Cooking Oil
- Non-oil components of the WCO were removed by separation using filter and moisture was removed by heating the oil at about 120°C for 30 to 45 minutes. Heating with electric heater is usually the easiest way to bring the oil up to required temperature. In order to determine the percentage of FFA in the oil, a process called titration is used. The vegetable oil is first mixed with methanol. Next, a mixture of Sodium Hydroxide (NaOH) and water is added until all of the FFA has been reacted. The purpose of mixing methanol and the catalyst (NaOH) is to react with two substances to form Methoxide. The amount of Methanol used should be 20% of the volume of the oil. Methanol and KOH are dangerous chemicals by themselves, with Methoxide even more so. None of these substances should ever touch skin. Vapours should not be inhaled. Gloves and ventilation are required at all times when working with these substances. Transesterification (bio-diesel reaction) is carried out. The methanol in excess is added to the oil in a beaker serving as a batch reactor. The mixture is then agitated for about 60 to 90 minutes and then left overnight for phase separation to take place due to gravity. After the transesterification reaction, one must wait for the glycerol to settle to the bottom of the container. This happens because Glycerol is heavier then bio-diesel. The settling will begin immediately, but the mixture should be left for minimum of 8 hours to 12 hours. After this, washing of Bio-diesel is done to wash out the remnants of the catalyst and other impurities. Generally water washing is preferred in which lukewarm water (about one third of raw bio-diesel) is added to raw bio-diesel, stirred for a short duration and then impurities are allowed to settle down at bottom with water. To produce bio diesel mechanical magnetic stirring is used. In this method, mixing of WCO and methanol is done in a tank equipped with mechanical stirrer. An electric motor is used to rotate the shaft around which blades are provided to stir the mixture of immiscible liquids (oil and alcohol are not miscible with each other), as shaft starts rotating a turbulence is created which disrupt the phase boundary between two immiscible liquid and thus resulted in proper mixing. Temperature is measured with the help of a thermometer and kept in the range of the 60-65°C. Biodiesel is then allowed for phase separation of methyl ester and glycerol in separating flasks. Fatty acid has higher specific weight therefore it will settle at bottom. Separation of methyl ester and glycerol will take 8 to 12 hr duration. After complete separation bio-diesel (methyl Ester) is visible in the upper layer and glycerol at the bottom. Bio-diesel is then separated from beaker for purification process and water washed. The catalyst present in the methyl ester is impurity. Excess methanol present in bio-diesel has been removed by vaporization process. To remove impurities and catalyst, water at around 40-50°C is mixed with the methyl ester and left for settling down. Water due to its higher specific gravity collected at bottom. Excess water is removed by heating the bio-diesel up to 100°C.
2.2. Production of Cashew Nut Shell Oil
- Extraction of CNSO from cashew nut shell includes open pan roasting, drum roasting, hot oil roasting, cold extraction, solvent extraction, super critical fluid extraction, pyrolysis process, Soxhlet extraction method. The percentage yield of oil varies with the type of extraction process. The Bio-Diesel is extracted from CNSO. It is done by making the CNSO to react with Methanol in the presence of catalyst like KOH which is Potassium Hydroxide. The procedure is to 100 ml of CNSO, 70 ml of methanol and 4gm of KOH is added in room temperature and is stirred well for one hour at room temperature itself. It will take 24 hours to get the bio-diesel. We get 100ml of bio diesel from 100 ml of CNSO.
2.3. Blending of WCO and CNSO with Diesel
- The produced biodiesels are blended with the regular diesel in different percentages. The blending process was carried out with the help of measuring jar and beaker. The approximate amount of diesel and biodiesel were added to the beaker and then transferred into the bottle. The bottles were shaking well and were allowed to stay upside down to ensure proper mixing of fuels. The bottle were stored in dry place and kept still for the 24 hours. Blends were checked for every 6 hours’ time intervals for any layer formation. All the blends were stable and passed the 24 hours. Blending is done for both WCO and CNSO with diesel with percentage of 10, 20, 30 and 40.
2.4. Investigation of Properties
- The following properties are found for blends of WCO and CNSO with diesel. Calorific value: A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is burning, it will heat up the surrounding air, which expands and escapes through a tube that leads the air out of the calorimeter. When the air is escaping through the copper tube it will also heat up the water outside the tube. The change in temperature of the water allows for calculating calorie content of the fuel.Viscosity: The redwood viscometer consists of vertical cylindrical oil cup with an orifice in the centre of its base. The orifice can be closed by a ball. A hook pointing upward serves as a guide mark for filling the oil. The cylindrical cup is surrounded by the water bath. The water bath maintains the temperature of the oil to be tested at constant temperature. The oil is heated by heating the water bath by means of an immersed electric heater in the water bath; Provisions is made for stirring the water, to maintain the uniform temperature in the water bath and to place the thermometer to record the temperature of oil and water bath. The cylinder is 47.625 mm in diameter and 88.90 mm deep. The orifice is 1.70 mm in diameter and 12 mm in length, this viscometer is used to determine the kinematic viscosity of the oil. From the kinematic viscosity the dynamic viscosity is determined.Fire Point: In the Pensky–Martens closed-cup flash-point test, a brass test cup is filled with a test specimen and fitted with a cover. The sample is heated and stirred at specified rates depending on the material that is being tested. An ignition source is directed into the cup at regular intervals with simultaneous interruption of stirring until a flash that spreads throughout the inside of the cup is seen. The corresponding temperature is its flash point.Fire Point: Pensky–Martens closed cup is sealed with a lid through which the ignition source can be introduced periodically. The vapour above the liquid is assumed to be in reasonable equilibrium with the liquid. Closed cup testers give lower values for the flashpoint than open-cup testers (typically 5–10 K) and are a better approximation to the temperature at which the vapour pressure reaches the "lower flammable limit".
3. Results and Discussion
- Results are taken for the blends of WCO and CNSO with diesel. Change in the density, fire point, calorific value for biodiesel and its blends are shown in the figure 1, figure 2 and figure 3 respectively. Kinematic viscosity at 30°, 40°, 50° and 60° for biodiesel and is blends shown in the figure 4, figure 5, figure 6 and figure 7.
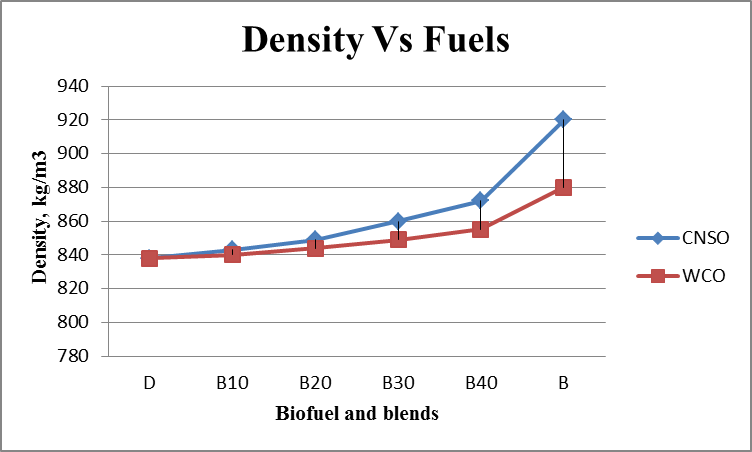 | Figure 1. Density Vs Fuels graph |
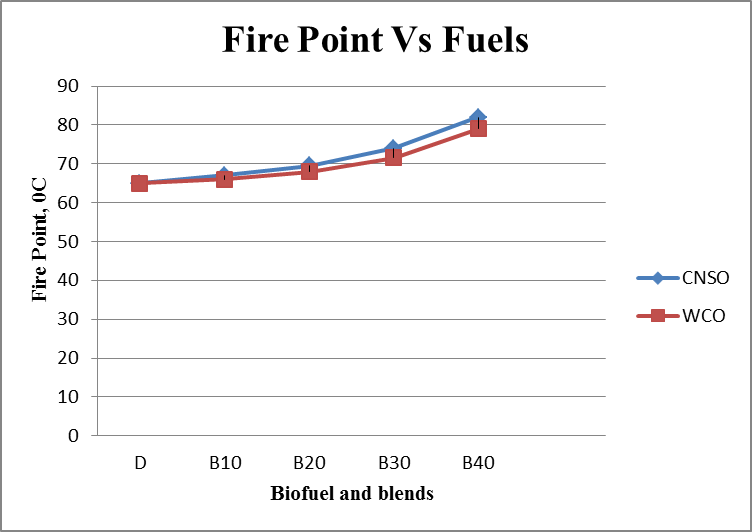 | Figure 2. Fire Point Vs Fuels graph |
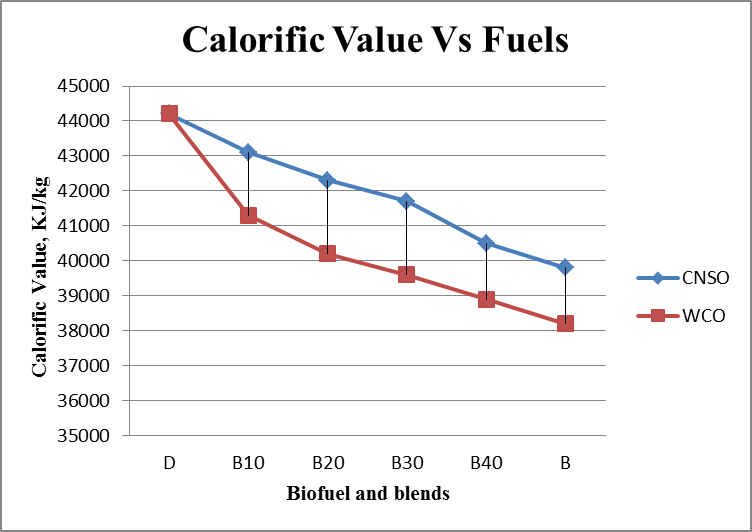 | Figure 3. Calorific value Vs Fuels graph |
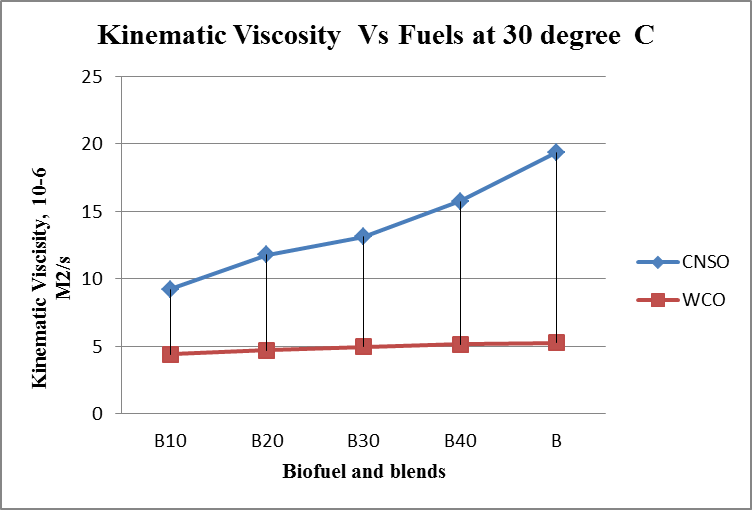 | Figure 4. Kinematic viscosity Vs Fuels graph at 30°C |
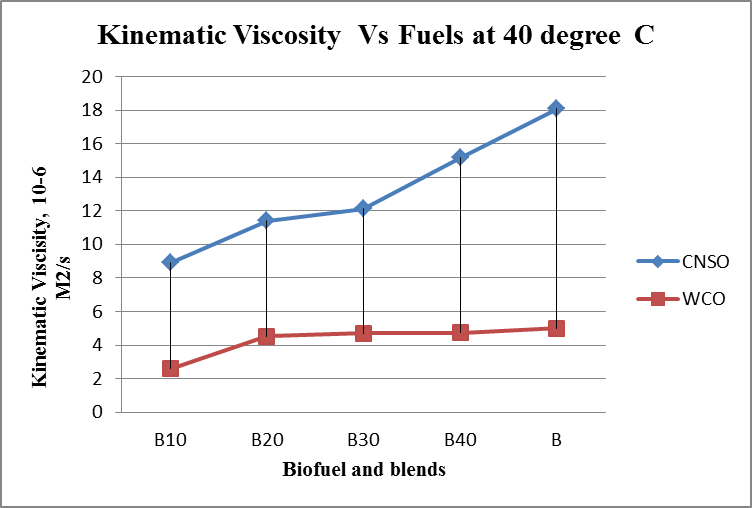 | Figure 5. Kinematic viscosity Vs Fuels graph at 40°C |
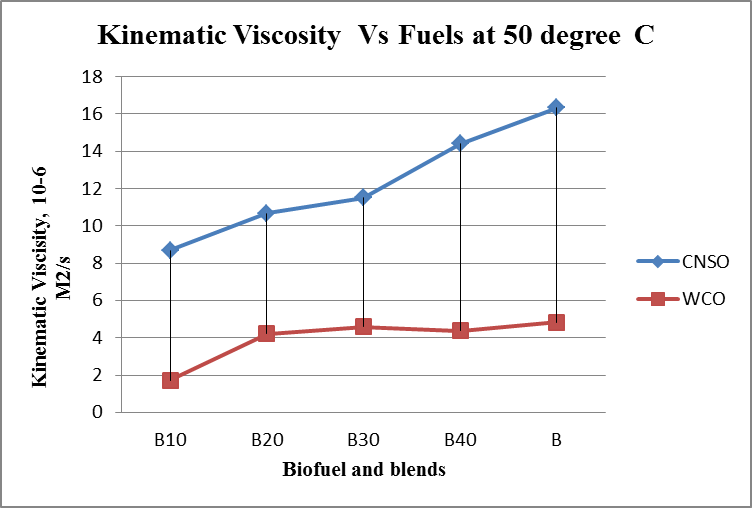 | Figure 6. Kinematic viscosity Vs Fuels graph at 50°C |
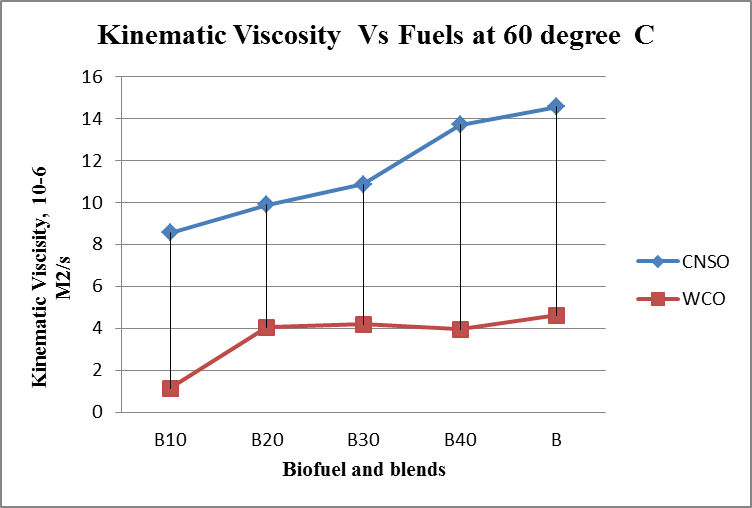 | Figure 7. Kinematic viscosity Vs Fuels graph at 60°C |
4. Conclusions
- Biofuels from Waste Cooking Oil and Cashew Nut Shell Oil are produced using different processes. Both WCO and CNSO properties satisfies ASTM standards. So Blending is done directly without any additional process. Comparison of properties of WCO and CNSO shows CNSO is more reactive than the WCO. With the increase in the temperature little change in the kinematic viscosity is observed for WCO. WCO can be used for high temperature applications as lubricant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML