-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2017; 7(2): 37-40
doi:10.5923/j.ep.20170702.01

Biogas Production from Blends of Cow and Fowl Dung Using Locally Made Anaerobic Digester
Ukpabi Chibueze F.1, Ntiwunka Okechukwu K.1, Emole Eke C.2, Ndulaka Justina2, Nwachukwu Ifeanyi3, Esenamunjor Clement3
1Department of Biochemistry, Abia State Polytechnic, Aba, Nigeria
2Department of Microbiology, Abia State Polytechnic, Aba, Nigeria
3Department of Mechanical Engineering, Abia State Polytechnic, Aba, Nigeria
Correspondence to: Ukpabi Chibueze F., Department of Biochemistry, Abia State Polytechnic, Aba, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

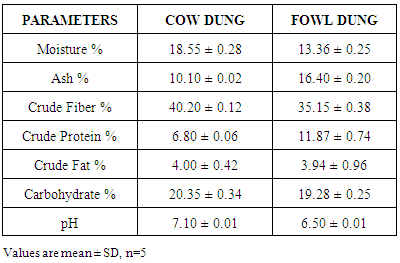
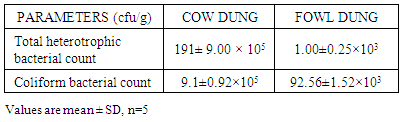
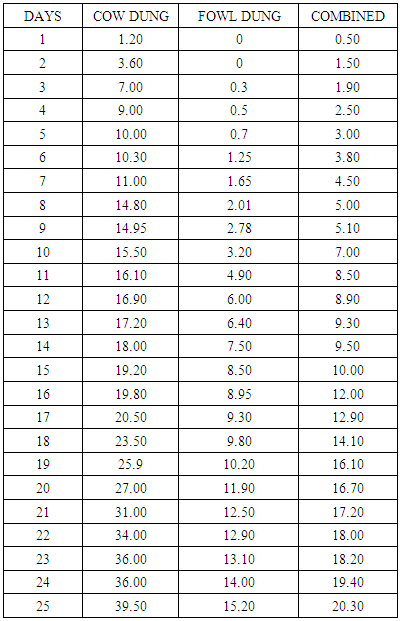
In Nigeria, million tones of animal dung are produced every year which can be utilized for better purposes. Hence anaerobic digestion becomes a promising technology. The project was to construct an anaerobic processing facility to generate biogas which will be more cost effective and economically friendly. The steel made biogas facility was provided with suitable arrangements for feeding, gas collection and draining residues. The gas outlet device was used to record the gas production arrangement. Fresh cow and fowl dung were collected from Aba North LGA slaughter house and Abia State Polytechnic poultry farm respectively. The proximate and microbial parameters of the dung were determined. The proximate analysis showed that the energy yielding nutrient values of the cow dung were significantly higher than the fowl dung. Similarly the heterotrophic and coliform bacteria were also higher in the cow dung than fowl dung. The data showed the volume of biogas production in respect of number of days under various experiments. It could be observed from the data that the volume of biogas production was higher in the cow slurry (39.50ml), followed by combined slurry (20.30ml) and then fowl slurry (15.30ml). The proximate and microbial composition of the cow dung may explain reasons for the bioefficacy of the cow dung thus justifying its usage.
Keywords: Cow dung, Fowl dung, Anaerobic digestion and Biogas production
Cite this paper: Ukpabi Chibueze F., Ntiwunka Okechukwu K., Emole Eke C., Ndulaka Justina, Nwachukwu Ifeanyi, Esenamunjor Clement, Biogas Production from Blends of Cow and Fowl Dung Using Locally Made Anaerobic Digester, Energy and Power, Vol. 7 No. 2, 2017, pp. 37-40. doi: 10.5923/j.ep.20170702.01.
Article Outline
1. Introduction
- Biological degradation of organic material under aerobic and anaerobic conditions is a key process within the natural metabolism of ecosystem [1, 2]. This term is often used in relation to energy, waste management and is now commonly associated with the anaerobic generation of methane. Anaerobic process could either occur naturally or in a controlled environment such as in a biogas plant [3]. Organic waste such as livestock manure and food waste are put on an airtight container called digester [4-6]. Livestock manure has been established as major source of microbes and also as a source of biogas production [7, 8]. It is a controlled ecological degradation process which allows efficient capturing and utilization of biogas (approximately 60% methane and 40% carbon dioxide) for energy generation [9]. In recent times varied technological modifications and improvements have been introduced to increase the efficiency of biogas production. Suyog [10] proposed a common way of preventing instabilities in start-up and avoiding acidification is to keep the organic load of the digester far below its maximum capacity. Similarly different methods have been developed to increase speed of fermentation for the bacteria gas producers, introduction of inoculums, the use of food and animal wastes [11, 12]. Energy generation operating cost could be reduced to the point that in the current economic framework small anaerobic digestion facilities can be mounted.Changes in microbial diversity may result in changes in dung function. Biogas can be measured by counting anaerobic microbes and/ or the amount of methane that are produced. Microbial diversity can be categorized into three groups [13]. Genetic analysis relates to the base structure of the community i.e the “library” of fundamental information that is present. Phenotypic analysis involves the prevailing expression of the genetic background which is the “living form” of the community. Functional analysis relates to the various processes the community is engaged in or potentially capable of carrying out such as could be revealed in a culture method. Genetic and phenotypic methods are advanced molecular techniques. They are not an economic option for poor rural dwellers in developing countries due to poor biotechnology facilities. The methanogenic bacteria belong to a group of bacteria with heterogeneous morphology. Heterotrophic bacteria derive their energy from the oxidation of already formed organic molecules. Heterotrophic bacteria require acid-producing bacteria and energy source for supporting their existence. Acid-producing bacteria create an atmosphere with ideal parameters for methane producing bacteria [7]. The organic carbon however has to be biodegradable but if the organic carbon is persistent, even when it occurs in large quantity, the heterotrophic will be low.The biodegradability of dung is indicated by biogas production or methane yield percentage of solids that are destroyed in the anaerobic digestion [1]. The biogas or methane yield is measured by the amount of biogas or methane that can be produced per unit of total solids contained in the dung after subjecting it to anaerobic digestion for a sufficient amount of time under a given temperature [1, 11].In Nigeria, million tones of animal manure are produced every year. Using anaerobic digestion, animal wastes and other biodegradable wastes can be utilized to achieve an environmental friendly state. This technology functions as a waste disposal system particularly for animal wastes and can therefore prevent potential sources of environmental contamination and the spread of pathogen and disease causing bacteria [3, 9, 12]. Anaerobic digestion of animal dung is achievable but different types of dung, results in varying degrees of biogas yield, thus the effects of mixing various types of dung should be determined. In this study, attempt was made to evaluate the efficiency of biogas production from single and combined dung using a constructed steel digester.
2. Materials and Methods
- Raw materialsThe raw materials used for this study were cow (CD) and fowl (FD) dung. Fresh cow and fowl dung were collected from Aba North LGA slaughter house and of Abia State Polytechnic Poultry Farm respectively. The dung represents an important source of micro organism as it contained the required microbes for anaerobic digestion. Proximate analysis and pH determinationProximate composition of the dung was carried out according to the method of AOAC [14] as described in Ukpabi et al [15]. The dung pH was determined electrometrically using glass electrode pH meter. The pH was measured at the ratio of 2:1 dung/water suspension. Microbial AnalysisPrevalence of dung bacterial species was determined using various and appropriate culture media for different organisms. The organisms include; (1) total heterotrophic bacteria, (2) coliform bacteria. The bacterial count was determined using pour plate method with appropriate medium. One drop (0.1ml) of the dung suspension from an appropriate tenfold serial dilution was inoculated into the plates in three replicate. Visible discrete colonies in the incubated plate were counted and expressed as colony forming units per gram (cfu/g) of dung sample.Biogas ProductionThe dung was sorted to separate domestic and plant materials and were mixed with water thoroughly by hand. The dung were weighed and poured into the digester. After the digester was kept for that day and gas production was checked the next day.The experimental studies were carried out in fermentation batches using the fabricated digester. In the single dung experiment, the digester was fed with 150g of cow dung and 150g of fowl dung mixed with water at a ratio of 1:1 respectively. In combined dung experiment, 150g of the mixed dung was introduced into the digester at the same ratio. The digester was provided with suitable arrangements for feeding, gas collection and draining residues. The digester was connected to a calibrated measuring cylinder with paraffin oil displacement arrangement to measure the volume of biogas produced. The slurry was allowed to ferment anaerobically for 25 days under mesophillic temperature of 26-38°C.
 | Figure 1. Diagram of the Constructed Digester |
3. Result and Discussion
- Organic material can be degraded aerobically or anaerobically. The methane producing bacteria utilize energy yielding nutrients for efficient biogas production. The proximate composition of the dung revealed the presence of energy-yielding nutrients at varying concentrations. Though the proximate composition of the cow dung was a little higher than fowl dung, thou there was no significant difference between the values except in protein. Protein values are significantly higher in the fowl dung than the cow. This may indicate that acids mostly from protein may cause decrease in pH of slurry. This has demonstrated that using feedstock with moderate caloric and nutritive values, microbes can be sustained for efficient biogas generation.
|
|
|
4. Conclusions
- It has been shown in this study that locally fabricated anaerobic digester can be used to produce biogas, using animal dung like cow and fowl dung. The experimental data obtained signified that high caloric feedstock with high carbohydrate and low fats contents could yield more biogas than other materials.
ACKNOWLEDGEMENTS
- The authors are grateful to the management of Abia state polytechnic, Aba and Tertiary Education Trust Fund (TETFUND), Nigeria for sponsoring this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML