-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2016; 6(1): 21-27
doi:10.5923/j.ep.20160601.03

Balanites Roxburghii Plant Oil as Potential Non-Edible Feedstock for Biodiesel Production
Priyanka Khanvilkar1, Grishma Patel1, P. S. Nagar2, Shailesh N. Shah1
1Bio-Energy Research Group, Chemistry Department, Faculty of Science, The M. S. University of Baroda, Vadodara, India
2Botany Department, Faculty of Science, the M. S. University of Baroda, Vadodara, India
Correspondence to: Shailesh N. Shah, Bio-Energy Research Group, Chemistry Department, Faculty of Science, The M. S. University of Baroda, Vadodara, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Plant oils from Ratanjyot (Jatropha curcas), Karanj (Pongamia pinnata), Neem (Azadirachta indica), and Mahua (Madhuca longifolia var. latifolia) etc. have been extracted and exploited as non-edible feedstock for the bio-diesel production in India. Our efforts have been converted to find area specific non-edible feedstock. With this point, Balanites roxburghii (BR) plant with high oil content, located at semiarid perennial plant and seed yield 2.5 tons/acre in third year to that 8.5/acre in 7th year under non-irrigated condition, was collected in the local area around Vadodara Gujarat India. The present work reports the synthesis of Balanites roxburghii methyl ester (BRME) from Balanites roxburghii oil (BRO) which has been extracted via a process called Hot Soxhlet Extraction process. The production process of BRME was carried out in two-step (1) Esterification of BRO in the presence of a homogeneous acid catalyst to lower the higher acid value. (2) Trans-esterification is carried out using CH3ONa as catalyst. BRME was then analysed by spectroscopic techniques such as FT- IR, C13 /H1 NMR and Gas Chromatography (GC) for analysis of fatty acid methyl ester and the fuel properties such as Density (ρ), Kinematic viscosity (KV), Acid Value (AV), Iodine value (IV), Cloud point (CP), Pour point (PP), Flash point (FP), Lubricity and Bromine number (BN) were determined. Lastly the comparative studies was carried out, of the FA profile as well as fuel properties of BRME with Balanites aegyptiaca methyl ester (BAME) and standard Soya beam methyl eater (SME) and the fuel properties of BRME was also compared with ASTMD6751.
Keywords: Non-Edible, Balanites roxburghii methyl ester, Extraction, Oil, Transesterification, Bio-diesel, Fuel Properties
Cite this paper: Priyanka Khanvilkar, Grishma Patel, P. S. Nagar, Shailesh N. Shah, Balanites Roxburghii Plant Oil as Potential Non-Edible Feedstock for Biodiesel Production, Energy and Power, Vol. 6 No. 1, 2016, pp. 21-27. doi: 10.5923/j.ep.20160601.03.
Article Outline
1. Introduction
- For the last 200 years fossil fuel consumption has considerably increased, which has led to several economic, political and environmental problems. The world has faced a sudden trauma regarding the energy depletion and environmental degradation [1]. The United States is one of the largest consumers of fossil fuels in the world [2]. Amongst the bio-fuels the most commonly being developed and used currently is bio-diesel [3-5]. Bio-diesel consists of long-chain alkyl esters made from virgin, used vegetable oils (both edible and non-edible) and animal fats. It has been found suitable for use as a blending bio-diesel fuel in diesel engines [6]. India is the most abundant resources of non-edible plant which could be utilized for the biodiesel production. Biodiesel is one of the most attractive alternative fuels due to it being an environmentally friendly fuel. It can be prepared from edible as well as non-edible feed stocks. At 15% growth per year utilization of biofuels has become one of the fastest growing markets all over the world [7]. There are many Non-Governmental Organizations (NGO) which deals with biofuels as one of the best renewable technologies that can reduce the dependence on Petro-diesel and to avert the worst of climatic change [8]. The importance of BIODIESEL may be summarized as the following:Ÿ Environmentally friendlyŸ A clean burning renewable fuelŸ No engine modification requiredŸ Increase in engine life Ÿ Bio degradable Ÿ Non-toxicŸ Easy to handle and store. The production of biodiesel from edible oils like Sunflower, Cynara Cardunculus, Awala Hirda seed, Soybean, Rapeseed, Sunflower, Sesame, Canola, Palm etc. has already been studied and reported [8]. The production of biodiesel from non-edible feed-stock such as Ratanjyot (Jatropha curcas), Karanj (Pongamia pinnata), Neem (Azadirachta indica), and Mahua (Madhuca longifolia var. latifolia) etc. were very commercially viable compared to edible oils. However, production of biodiesel from edible oil crops is not desirable. Latterly, there were many concerns business organization involving the use of food crops as a feedstock for fuel production. The use of crops for energy has caused the food to competent with each other in many ways (agricultural land, skilled labor, water, fertilizers, etc.) Additionally, the high price of biodiesel derived from food grade vegetable oils makes it difficult to compete economically with the fossil based industries. A cost effectiveness and non-food grade vegetable oil is a powerfull feedstock for production of bio-diesel [9-10]. The biodiesel obtains, must be satisfied according to accepted fuel standards such as ASTM D6751 in the United State or the Committee for Standardization (CEN) standard EN 14214 in Europe before combustion in compression-ignition (diesel) engine [11].Our continuous efforts have been converted to find area specific non-edible feed stocks. Hence, with this in view, Balanites roxburgh (BR) plant, Balanites (Greek word: acorn-shaped) is an important genus of thorny shrubs or trees distribute in dry regions of India, Africa, Syria and Arabia. Balanites roxgurghii tree is a member of the family Zygophyllaceae [12]. It is locally known as hindota/hiran, which comes in various sizes from small to large and are an evergreen scraggy shrub. The small thorny tree deciduous with an erect, short trunk and ascending branches are about 8 meters long. Other alternative leaves are Caracus, which is flowers that are regular, bisexual, drupe fly shy, ovoid and only one seeded [11]. The objective of the current research was to prepare Balanites roxgurghii methyl ester (BRME) from Balanites roxgurghii oil (BRO) via trans-esterification process using an alkaline catalyst and to compare their chemical composition (fatty acid profile) and fuel properties to the biodiesel fuel standard ASTM D6751, EN 14124 as well as with Soy bean oil methyl ester (SME) and with Balanites aegyptiaca [13] methyl ester (BAME) as reported.
2. Materials and Methods
2.1. Materials
- The Balanites roxburghii trees were situated in the region named as Kissannagar, near Dabhoi village,Vadodara district, Gujarat state, India. The fruits of Balanites roxburghii were collected from fully grown trees, in summer season. Approximately 2 Kg fruits were collected. They were socked in containers of clean water and were boiled for 4 hours, then were left to cool down. The epicarp and glycoside pulp of the fruits were taken out and the nuts were washed with fresh water and air dried for 4 days in the summer time. The nuts were then cut and kernels were out. Using these BR kernels further oil extraction operation was carried. The solvent used like petroleum ether, Hexane, methanol and the catalyst Sodium methoxide used for preparation BRME have been purchased from SRL (Sisco Research Laboratories Pvt. Ltd. Mumbai, India) and Sigma Aldrich.
2.2. Balanites Roxburgh Oil (BRO) Extraction
- The process of extraction of BRO from BR kernels was carried out using the method as reported earlier by our research group [12]. The BRO extracted using this method was further used for synthesis of BRME.
2.3. Pre-treatment of BRO
- The major problems due to the high value of free fatty acid (FFA) are (1) a higher quantity of catalyst needed to be used. However, this was not cost effective and expensive. (2) Soap (fatty acid salt) is formed, which made the washing of the finished product more difficult to obtain. (3) The formation of water as by-product delayed the reaction. (4) The FFAs was not converted into biodiesel, which reduced the yield and also increased the acid value of the biodiesel. The percentage of free fatty acid (FFA) in BRO was of a relatively high value, AV of 4.3 mg KOH/g (FFA content = 2.1 wt. %). Hence, it was necessary to reduce FFA value of BRO before subjecting the oil for trans-esterification process. In order to eliminate the higher FFA content in the BRO, the following steps were carried:At first the esterification step was carried out. Take 5.70g BRO was poured in a 25ml rounded bottom flask. Later on, around 2.4g methanol was added. Afterwards the catalytic amount of H2SO4 was added. The reaction was kept under the gentle reflux for 3 h. Then the reaction mixture was washed 3 times with 5% (W/V) sodium bicarbonate solution. The oil phase was dissolved in hexane, kept over magnesium sulphate for 2 hours, filtered and finally the volatiles were removed under vacuum and then the BRO obtained, was ready for the trans-esterification process.
2.4. Preparation of Balanites Roxburghii Methyl Ester (BRME)
- For the first step BRO obtained take 100 ml of a one-necked round bottom flask equipped with a water condenser and a magnetic stirrer. Then 20 g of BRO was taken, CH3OH to oil molar ratio was taken 4.5:1.0 using 1 wt.% CH3ONa solution as catalyst. The whole reaction mixture was vigorously stirred for 2 hours at 60°C temperature under 600 rpm under reflux condition. The progress of the reaction was monitored by TLC (Thin layer Chromatography). After completion of the reaction, the mixture turned yellowish in colour. The whole reaction mixture was then transferred into a separating funnel. Hexane and water were also added to the reaction mixture that resulted in the formation of emulsion. Hence, in order to avoid this, brine solution was added in a separating funnel and was shaken thoroughly. The two layers separated: the upper organic layer was of BRME while the lower layer aqueous layer contained water, glycerol, and unreacted methanol. In the organic phase, dried anhydrous sodium sulphate was collected. The BRME yielded 34% was then characterized by the spectroscopic methods such as C13 / H1 NMR, FT-IR, GC and by other fuel properties such as: Density (ρ), Kinematic viscosity (KV), Acid Value (AV), Iodine value (IV), Cloud point (CP), Pour point (PP), Flash point (FP), Lubricity and Bromine number (BN).
3. Equipment and Methods
3.1. Fatty Acid Profile by GC
- The BRME synthesized from BRO [12] was analysed for fatty acid composition using the Electron Impact Ionization (EI) method on an auto system XL gas chromatography (Perkin Elmer instrument, Germany). The capillary column (30m × 0.25μm × 0.25μm) was used. The detector temperature was programmed at 240°C with the flow rate of 0.8 ml/min. The injector temperature was set at 240°C. Nitrogen was used as a carrier gas. The designation of peaks were obtained by retention times of known reference standards. BRME determine were run in duplicate and mean values have been reported. (Sophisticated and Instrumentation Centre for Applied Research and Testing, SICART laboratory, Vallabh, Vidyanagar, Gujarat, India).
3.2. Nuclear Magnetic Resonance (NMR)
- 1H NMR and 13C spectra were recorded on a Bruke AR X 400 Spectrometer at 400 MHz using CDCl3 as solvent.
3.3. Fourier Transform- Infrared (FTIR) Spectrum
- The FTIR spectrum was performed on a Shimadu-8400S Spectrophotometer by a neat method. The spectra were obtained over the frequency ranged from 4000-400 cm-1 at a resolution of 4 cm-1.
3.4. Density
- The density (ρ) which is normally expressed as a specific gravity defines the ratio of the mass of a volume of the fuel, compared to the mass of the same volume of water [13]. The density was determined by a general method. In a measuring cylinder, 10 ml BR oil was poured and weighed. It was calculated by the equation shown below:
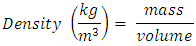
3.5. Kinematic Viscosity
- The viscosity that measures the fluid resistance to flow is called Kinematic viscosity [13]. (Ʋ, mm2/s) which was measured at 400C using Stabinger Viscometer SVM 3000 as as per ASTM D7042. The experiment was run in triplicate and mean values are reported. [14].
3.6. Acid Value
- Acid value of BRME was determined according to the literature method mention in earlier studies. [12] the titration’s end point was determined by colour change to pink and visually using phenolphthalein indicator. The sample was run in triplicate and average were reported.
3.7. Iodine Value
- Iodine value (g I2 / 100g) was calculated from the fatty acid profile according to AOCS official method of 1c-85 (AOCS, 1999d). [15].
3.8. Cloud Point
- Cloud point (CP, C) determination was determined by using the ASTM D5773 method, by the model PSA-705 Phase Technology Analyser Cloud point, which was rounded to the nearest whole degree (°C) [16].
3.9. Pour Point
- Pour point (PP) determination was used in Lin Tech as per ASTM D97, respectively. All runs were carried out in the same manner. The sample temperature was measured in 3°C increments at the top of the sample to the highest point of the burette was achieved. [17].
3.10. Flash Point
- The flash point of the products was determined using Koehler Inc. apparatus as per ASTM D 93 method, this the lowest temperature at which a combustible mixture can be formed above the liquid fuel pattern.
3.11. Bromine Number
- The bromine number was determined using the ASTM-D1159 method. Bromine number is the measure of the amount of bromine in grams absorbed by 100 grams (3.5 oz.) sample. The number indicates the degree of unsaturation [18].
3.12. Lubricity
- Lubricity is “the property of a lubricant that causes a difference in fraction under the condition of boundary lubrication when all the known factors except the lubricant remain the same” [13]. Hence, lower the fraction, the higher the lubricity. HFRR (High Frequency Reciprocating Rig) wear scar, unit (μm), was determined using a PCS Instrument, ASTM D-6079. [19]
3.13. Average Molecular Weight of Vegetable Oils
- The average calculated molecular weight (MWcal, g/mole) was determined by a weighted average method by utilizing the FA profiles depicted in (table 3). The molecular weight of FA found in the vegetable oil was multiplied by its corresponding weight percentage as determined by GC [20]. The aggregate of these values (minus the acidic proton) was multiplied of 3 and the glycerol fragment (minus the oxygen atoms, as they were accounted for in the FA fragments) were added, which ensure in an average calculated MW of vegetable oil. For the sake of providing calculated MW values that were not artificially depressed [12].
4. Results and Discussion
4.1. FTIR Data Analysis
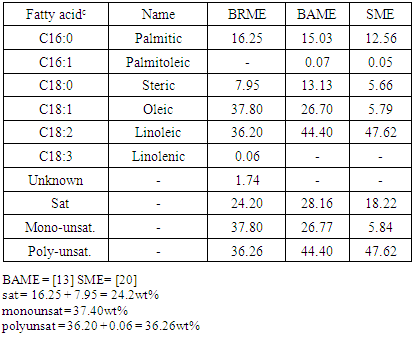
- The FT-IR spectra of BRO and FT-IR spectra of BRME were compared. The IR spectrum for BRO shows ester linkage of triglyceride at 1745 cm-1. From the spectra of BRME the ester linkage of Fatty acid Methyl ester at 1744 cm-1; the CO-O-CH3 stretching frequency is absent in BRO and seen in the BRME at a frequency of 1436 cm-1. The FTIR of BR oil with a base line of BRME clearly showed that the carbonyl group of the BR oil shifted from 1745 cm-1 to 1744cm-1. This indicates the fact that in BRME only one carbonyl group is present compared to the baseline of BR oil (which contains three carbonyl groups). (See table 1).
|
4.2. 13C and 1H NMR Data Analysis
- The BRME was synthesized from BRO and analysed by NMR. The highest peak for BRME was appeared δ = 1.4 (m) in the spectra (δ=chemical shift value), suggesting the presence of long fatty acid chain present in BRME. Second peak seems at δ = 3.6 (3H s) singlet representing the –OCH3 group. Indicating of the fact that BRO converts into BRME via Trans-esterification process.13C NMR, characteristic peaks were observed at 174.31 and 174.26 ppm (-O-CO-), several peaks in the range 130.20-127.91 ppm (-CH=CH-), 53.40 ppm (CH3-O-CO), 33.11, 33.09 ppm (both O-CO-CH2-), 32.93, 32.90,32.52 (all three-CH2-CH2-CH3), numerous peaks in the range 28.76-28.08 ppm (all- (CH2)x-), 26.20, 26.18, 26.16 (weak) ( all three –CH2-CH=CH-CH2-), 25.63, 24.86, 24.84 (both O-CO-CH2-CH2-), 22.68 and 22.54 ppm (both – CH2-CH2-CH3), 14.17 and 14.05 ppm (both –CH2-CH3).
4.3. Fatty Acid Composition and Free Fatty Content
- The FA profile obtained in BRME was oleic (C18:1; 37.80 wt%) acid and linolenic (C18:3; 0.06 wt%) acid with palmitic (C16:0 ; 16.25 wt%) acid, linoleic (C18:2; 36.20 wt%) acid also steric (C18:0; 7.95 wt%) acid were detected in significant quantities (Table 2). This result was compared with previous studies BAME [13]. The FA profile reported for BAME was oleic (C18:1; 26.70 wt%) acid with palmitic (C16:0 ; 15.03 wt%) and linoleic (C18:2; 44.40 wt%) and also steric (C18:0; 13.13 wt%). The average MW of BRME calculated from FA profile was 291.69 g/mole (Table 3). The degree of saturation in BRME (24.2 wt%) was lower than the degree of saturation in BAME. While the degree of unsaturation, mono unsaturation and poly unsaturation in BRME (37.80 wt%, 36.26 wt%) was comparatively high when compared with BAME (26.77 wt%, 44.4wt%). Thus, from the above study of FA profile, it can be said that the BRME and BAME were quite different in terms of the FA profile even though they belonged to the same family of Zygophyllaceae. It can be depicted from Table 3 that oleic acid was present in the sample. The BRME collected was higher (37.80 wt%) then present in the sample of BAME (26.70 wt%). Similarly, the composition varied in other saturated FA and unsaturated FA of both samples, especially linolenic acid. It had 0.06 wt% in the present study while it was totally absent in BAME. It can be seen that the main fatty acid in the BRME was oleic acid (37.80 wt%) followed by linoleic acid. (36.20 wt%). The main fatty acid in BAME was linoleic acid (44.40 wt%) followed by oleic acid (26.70 wt%) (see Table 2). The FA profile of BRME was also compared with the standard of SME sample in which the oleic (C18:1; 5.66 wt %) acid with palmitic (C16:0; 12.56 wt %) acid, linoleic (C18:2 47.62 wt %) acid and also steric (C18:0; 5.66 wt %) acid. The degrees of saturation and mono unsaturation were higher in BRME (42.2 wt %, 37.80 wt %) compared to the sample of SME (18.22wt %, 5.84 wt %). While poly-unsaturation was higher in SME (47.62 wt %) compared to BRME. By comparing all the three samples of methyl ester (BRME, BAME and SME), the overall degree of saturation was higher in BAME (28.16 wt %) than BRME (24.20 wt %) and SME (18.22 wt %). However, the degree of unsaturation (mono unsaturation / poly unsaturation) was higher in BRME (74.06 wt %) compared to BAME (71.17 wt %) and SME (53.46 wt %). (Table 2).
|
5. Fuel Properties of BRME
5.1. Molecular Weight
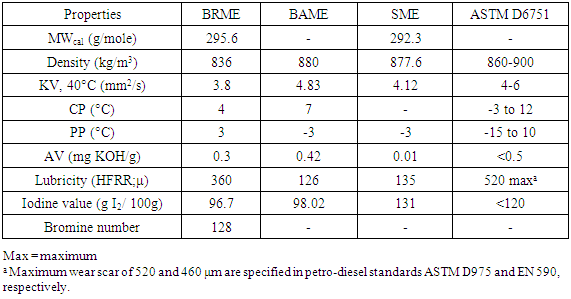
- The molecular weight of BRME was calculated using the fatty acid profile, which came out to be 295.60 g/mole, when compared to SME, which was about 292.33 g/mole. The overall saturation and unsaturation present in BRME was around 98.26 wt. % and in SME it was 71.68 wt. % (Table 2). Over all the FA composition in BRME was somewhat higher than the FA composition of SME that slightly increase molecular weight of BRME.
5.2. Kinematic Viscosity
- The kinematic viscosity of BRME (3.5 mm2/s) was lower than that of SME (4.12 mm2/s) and BAME (4.83 mm2/s) at 40°C (Table 3). The degree of poly unsaturation was higher in SME (47.62 wt. %) as well as in BAME (44.4 wt. %). Higher the level of unsaturation there will be increase in KV. The degree of unsaturation was lower in BRME (36.26 wt. %). So the KV was also quite lower than compare with BAME and SME and also KV of BRME is following in the range given by ASTM D6715.
|
5.3. Low Temperature Properties
- The low temperature operability of BRME was characterized by a PP of 3°C, which was higher than that obtained for SME -3°C and BAME -3°C. The CP of BRME was 4°C again lower than the CP of BAME 7°C (Table 3). The relatively high level of saturated FA contained in BRME (24.20 wt. %; Table 2) versus SME (18.22 wt. %; BAME 28.16 wt. % Table 2) was assign due to its heighten low temperature operability. The nature and amount of saturated FA composition decide the low temperature properties. An early study enlightened a reasonable connection between the saturated FA content and the low temperature properties. PP and CP values of BRME were following in the range mention in ASTM D6715 standard.
5.4. Acid Value
- The percentage of free fatty acid (FFA) in BRME was relative, 0.3 mg KOH/g (FFA content = 0.015 wt. %) (Table 3) and of SME was essentially free of FFA (AV 0.01 mg KOH/g). The FFA content is very important for the chemical modification which is conducted in the presence of a catalyst, which shows more sensitivity towards carboxylic acids [21]. The feedstock in which FFA content is greater than 0.5wt% would lower the amount of product yields [21]. Consequently, the acid-catalyser pre-treatment is required in order to lower the higher FFA content in BRO [12] (AV 0.37 mg KOH/g) [22].
5.5. Density
- Density of the BRME increase with increase in unsaturation. The density depends on the free space available within the molecules which in turns depend on the degree of unsaturation. From the FA composition the degree of unsaturation in BRME is lower than BAME and SME (table 2). So the density of BRME was lower (836 kg/m3) than density in BAME (880 kg/m3) and SME (877.6 kg/m3).
5.6. Iodine Value
- The Iodine value (IV) is calculated based on the fatty acid profile obtained. IV depends on the level of unsaturation present in sample. The IV in BRME sample was 96.4 g I2/ 100g and that of BAME was 98.02 g I2/100g, which was lower than the SME sample 131 g I2/ 100g; (Table 3) highest degree of unsaturated FA content higher will be IV. In BRME Unsaturated FA (36.26 wt. %) was quite more than in BAME (44.40 wt. %) and SME (47.62 wt. %). The BRME, Iodine value was also matching with ASTM D6715.
5.7. Lubricity
- The lubricity is not designated in ASTM D6715 but is admitted in the Petro-diesel standards ASTM D975 with maximum decreed wear scars of 520 and 460μm, respectively. Fuels with low lubricity can cause failing of diesel engine parts that bank on lubrication from fuels, such as fuel pumps and injector [2]. The wear scar got by BRME by the high-frequency reciprocating rig (HFRR) lubricity method ASTM D6079 (60°C) was 360μm and that of BAME 126 μm as well as SME 135 μm. As required, the lubricity of BRME was considerably under the maximum limits set forward in the aforesaid petro diesel.
6. Conclusions
- Determining a sustainable source of renewable energy is a worldwide undertaking task. After doing analysis of all possible methods for the conversion of Balanites roxburghii oil (BRO) into Bio-diesel (BRME) many important conclusions have been obtained. First, after doing a pre-treatment of BRO was done in order to lower FFA content, After lowering the FFA content up to certain values as mentioned in ASTM D6751 then the BRO was ready for the further use in synthesis of methyl ester (BRME) via single step process in presence base catalysed CH3ONa 1 wt. % called trans-esterification method. The BRME obtained from this method can be successfully be used as fuel in diesel engine as it meets specification in bio-diesel standards ASTM D6751. From the FA composition, the degree of unsaturation in BRME (36.2 wt. %) is quite lower than BAME (44.40 wt. %) and SME (47.62 wt. %). The good quality of bio-diesel has control short chain FA ester as well as reduce in double bond character i.e. lower unsaturation. As the degree of unsaturation decrease there is increase in cetane number were as decrease in NOx emission, smoke opacity and HC (Hydrocarbon) emission. More over after studying the FA profile and fuel properties like KV, PP, CP, IV etc. it has been concluded that BRME has all excellent properties that one present in a bio-diesel than compare to BAME and SME. The complete correspondence for production of bio-diesel from non-edible seeds will help in marketing the product and will also aid our economy by bringing down the import of crude petroleum.
ACKNOWLEDGEMENTS
- The authors highly thank the UGC for providing financial support and to Dr. Bharat Newalkar (Bharat Petroleum Corporation Limited, Central Noida, Uttar Pradesh.) and his group for giving us excellent support for the analysis on fuel properties.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML