-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2014; 4(1): 7-15
doi:10.5923/j.ep.20140401.02
Transesterification of Palm Oil by Using Silica Loaded Potassium Carbonate (K2CO3/SiO2) Catalysts to Produce Fatty Acid Methyl Esters (FAME)
R. Irmawati1, 2, I. Shafizah1, A. Nur Sharina1, H. Abbastabar Ahangar1, 3, Y. H. Taufiq-Yap1, 2
1Centre of Excellence for Catalysis Science and Technology, Faculty of Science, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
2Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
3Materials Science and Engineering Department, Islamic Azad University, Najafabad Branch, Najafabad 85141-43131, Iran
Correspondence to: R. Irmawati, Centre of Excellence for Catalysis Science and Technology, Faculty of Science, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Transesterification of palm oil with methanol to form fatty acid methyl esters (FAME) was performed, using silica loaded with potassium carbonate, K2CO3/SiO2 as a solid base catalyst. The catalyst was prepared by an impregnation method and calcined at 1237 K. The prepared catalysts were characterized by X-ray diffraction (XRD), Brunauer – Emmett - Teller (BET) surface area analysis, scanning electron microscopy (SEM), and temperature-programmed desorption of carbon dioxide (CO2-TPD). High yield biodiesel (98.10%) was obtained with a reaction time of 3 h, reaction temperature of 333 K, molar ratio methanol to oil of 20:1, catalyst loading of 20 wt%, and catalyst amount of 4 wt%. Proton nuclear magnetic resonance (1H-NMR) confirmed the existence of FAME with distinct peaks equivalent to hydrogen singlet from the methyl ester methoxyl group at 3.67 ppm and from methylenic hydrogen at 2.31 ppm. FAME was also successfully quantified using gas chromatography (GC) where peaks corresponded to fatty acid methyl esters of palm oil.
Keywords: Biodiesel, Transesterification, Base catalyst, Palm oil
Cite this paper: R. Irmawati, I. Shafizah, A. Nur Sharina, H. Abbastabar Ahangar, Y. H. Taufiq-Yap, Transesterification of Palm Oil by Using Silica Loaded Potassium Carbonate (K2CO3/SiO2) Catalysts to Produce Fatty Acid Methyl Esters (FAME), Energy and Power, Vol. 4 No. 1, 2014, pp. 7-15. doi: 10.5923/j.ep.20140401.02.
Article Outline
1. Introduction
- Awareness of environmental protection encourages many researchers to study in biodiesel production as an alternative to fossil fuels. Fossil fuel can give negative impact on the environment through carbon oxides emissions, unburned hydrocarbons, sulphur dioxide and particulates [1,2,3,4]. Biodiesel can minimize greenhouse gas emissions because carbon dioxide from biodiesel combustion is offset by the carbon dioxide sequestered while growing oil palm or other feedstock [5]. Biodiesel usually obtained from vegetable oil such as palm oil [6], soybean oil [7], canola oil [8], and rapeseed oil [9] or sometimes from animal fats [10] by transesterification (Scheme 1) and molecules in biodiesel are primarily known as Fatty Acid Methyl Esters (FAME). Triglyceride are the composition of vegetable oils and animal fats, which are esters containing three free fatty acids. During transesterification, triglycerides are reacted with methanol which is low molecular weight alcohol to produce methyl esters of fatty acids and glycerol.Triglycerides to methyl esters in complete reaction involves three sequential reactions with a monoglyceride (MAG) and a diglyceride (DAG) as intermediates. During transesterification, triglycerides (TAG) in the oil react with alcohol in the presence of a catalyst such as sodium hydroxide, NaOH or potassium hydroxide KOH to produce biodiesel. Transesterification occurs in three sequential reversible steps: (a) TAG reacts with methanol to produce a diglyceride (DAG), liberating a single fatty acid methyl ester, (b) DAG reacts with methanol to produce a monoglyceride (MAG) and another FAME, and (c) MAG reacts with methanol to produce a FAME, liberating the glycerol by-product. However, MAG and DAG are formed and remain in the final biodiesel product [11].
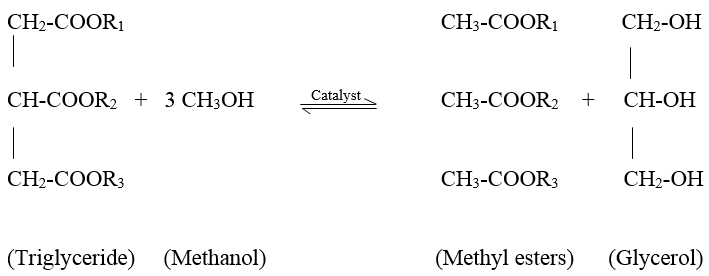 | Scheme 1. Transesterification |
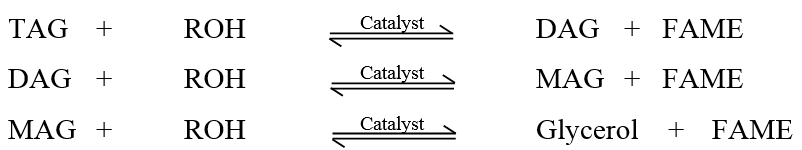 | Scheme 2. Three consecutive reactions during transesterification |
2. Materials and Method
2.1. Materials
- Refined palm oil was purchased from Giant Hypermarket, Malaysia and all chemicals such as potassium carbonate (99.99%), silica (99.99%), and methanol were obtained from Merck, Germany. The given properties of silica were pore size of 100 Å and surface area 330 m2/g, making it suitable for use as a catalyst support.
2.2. Methods
2.2.1. Catalyst Preparation
- The catalysts were synthesized by using a wet impregnation method. Potassium carbonate powder, K2CO3 was mixed with 10 mL deionized water to form a K2CO3 solution. The solution was poured onto 5 g silica which acted as a support for the catalyst with weight percentage of 5 wt%. The mixture was stirred for 30 min. The solids were dried overnight in an oven at 373 K and calcined in air at 1273 K for 4 h. The calcined catalyst was then crushed into powder and sieved. The preparation process was repeated for other catalyst loadings of 10 wt%, 15 wt%, and 20 wt% K2CO3/SiO2.
2.2.2. Catalyst Characterization
- The crystalline phase of the catalyst was assessed by X-ray diffraction (XRD) analysis using a Shimadzu XRD-600 Diffractometer by employing CuKα radiation (λ = 1.541 Å) generated by Philips glass diffraction x-ray tube broad focus 2.7 kW type, operated at ambient temperature (30 kV and 100 mA). The samples were scanned at a range of 2θ = 10o – 60o with a scanning rate of 2o/min. The obtained diffractograms were matched against the Joint Committee on Powder Diffraction Standards (JCPDS) PDF1 database version 2.6. The surface area of the samples were determined by the nitrogen (N2) adsorption-desorption technique, using Thermo Finnigan Sorptomatic Instrument model 1900. The BET surface area was calculated and total pore volume was determined by the estimation from the N2 uptake
 The morphology and surface structure of the sample (support and catalysts) were identified using SEM with LEO 1455 Variable Pressure scanning electron microscopy. The analysis was performed at an accelerated voltage of 20kV. The samples were coated with a thin layer of gold as the conducting material using a BIO-RAS sputter coater. Basicity of the catalysts was determined using CO2-TPD analysis and was performed using Thermo Finnigan TPD/R/O 1100. For each experiment, 0.002 g of catalyst was pre-treated in nitrogen to remove all water vapour and any impurities in the pipeline, which was then heated up to 523 K at 30 K min-1. The samples were cooled to adsorption temperature 300 – 450 K and loaded with carbon dioxide. Prior to analysis, the pre-treated samples were flushed with helium and heated up to 1173 K at 10 K min-1.
The morphology and surface structure of the sample (support and catalysts) were identified using SEM with LEO 1455 Variable Pressure scanning electron microscopy. The analysis was performed at an accelerated voltage of 20kV. The samples were coated with a thin layer of gold as the conducting material using a BIO-RAS sputter coater. Basicity of the catalysts was determined using CO2-TPD analysis and was performed using Thermo Finnigan TPD/R/O 1100. For each experiment, 0.002 g of catalyst was pre-treated in nitrogen to remove all water vapour and any impurities in the pipeline, which was then heated up to 523 K at 30 K min-1. The samples were cooled to adsorption temperature 300 – 450 K and loaded with carbon dioxide. Prior to analysis, the pre-treated samples were flushed with helium and heated up to 1173 K at 10 K min-1. 2.2.3. Transesterification Reaction
- Synthesized catalysts were tested for transesterification of palm oil. Commercial palm oil (cooking oil Cap Buruh) was used and the experiments were performed in 50 mL round bottom flasks with a water-cooled condenser. 5 g of palm oil, methanol (MeOH) to oil molar ratio of 20:1, catalyst amount of 4 wt% with 5 wt% K2CO3/SiO2 was put into the round bottom flask. Reflux was performed at 333 K for 1 h. The experimental design consisted of five factors and four levels (Table 1). At the end of the reaction, the mixtures were centrifuged. Three phases formed, the upper layer was methanol, the middle layer was a mixture of biodiesel and little amount of glycerol, and the bottom layer was solid catalyst. The catalyst was easily removed after centrifugation. Methanol was then removed from the biodiesel mixture by heating the mixture at 343 K for 1h to completely evaporate the methanol. The biodiesel mixture was left overnight for self-separation of biodiesel from glycerol. A separating funnel was used, resulting in an upper layer of biodiesel and bottom layer of glycerol. The biodiesel obtained was analyzed using 1H-nuclear magnetic resonance (1H-NMR) and gas chromatography (GC).
|
3. Results and Discussion
3.1. Characterization of the Catalyst
3.1.1. X-ray Diffraction (XRD)
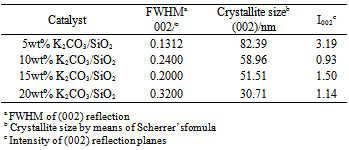
- The XRD pattern of SiO2 support (Figure 1) shows three main peaks at 2θ = 20.31o, 21.50o, and 35.5o which correspond to (100), (002), and (110) planes, respectively. All of these peaks are well matched to the reference peaks from SiO2 references peaks from SiO2 JCPDS file no. 18-1169. However, the peak at 2θ = 23.01o is not clearly seen. This is due to the semicrystalline nature of the calcined SiO2 support resulting from the irregular arrangement of atoms and/or molecules. Impregnating the pure SiO2 solid with 5wt% K2CO3 produced the effective progressive increase in the degree of crystallinity of the above mentioned phase and intensity. The appearance of peak at 2θ = 23.01o is proportional to the amount of K2CO3. The presence of K2CO3 was observed according to the JCPDS file no. 01-07-0292. Table 2 lists the full width at half maximum (FWHM) values of the catalyst corresponding to 2θ = 21.50o of the (002) plane. It is found that the FWHM values increases with K2CO3 loading. It can be seen from Figure 1 that the peak intensity decreases suggesting that at low loadings, K2CO3 might be mainly residing on the catalyst surface. With increased amounts, K2CO3 might also be incorporated into the SiO2 lattice causing disarray in the support atomic arrangement. The K2CO3 not only disrupts SiO2 crystallinity but also affects the size of the particles. The crystallite sizes of K2CO3/SiO2 corresponding to 2θ = 21.50o were 82.39, 58.96, 51.51, and 30.71 nm with K2CO3 loadings of 5, 10, 15, and 20 wt% K2CO3/SiO2, respectively. The crystallite size of SiO2 was calculated by using the Debye-Scherrer formula:
 | Figure 1. X-ray patterns of SiO2 and K2CO3 supported SiO2 catalysts ( ) K2CO3 ( ) SiO2 |
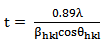 | (1) |
|
3.1.2. BET Surface Area Measurement
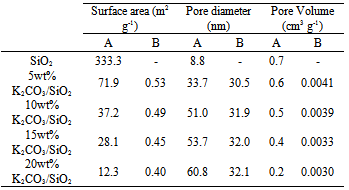
- The BET surface area, pore volume, and pore diameter of the supported catalysts are presented in Table 3. As shown in the table, the BET surface area of unsupported SiO2 is 333.3 m2/g. However, the surface area decreases significantly to 71.9, 37.2, 28.1 and 12.3 m2/g for 5, 10, 15, 20 wt% of K2CO3 loading, respectively. The BET surface area values further decreased when the samples were calcined, in which 0.53, 0.49, 0.45, and 0.40 m2/g were obtained for 5, 10, 15, 20 wt% supported samples, respectively. The huge differences in the BET surface area values for the calcined samples as compared to the uncalcined ones are due to the granulation of the originally powdery solid when the samples were calcined at high temperatures.
|
3.1.3. Scanning Electron Microscopy (SEM)
- Details of the surface morphology of the K2CO3/SiO2 catalyst were obtained from scanning electron microscopy (SEM) with magnification of 3000x and at a scanning voltage of 15kV are shown in Figure 2. All of the supported catalysts showed similar morphology and there is no particular shape can be derived from the image except for the rough surfaces of the materials. The images also shows
 were well distributed on SiO2.
were well distributed on SiO2.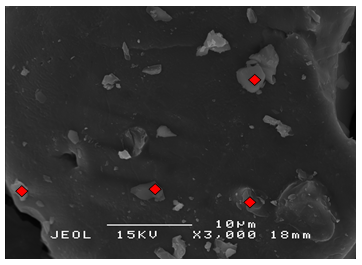 | Figure 2(a). SEM micrograph of 5 wt% K2CO3/SiO2 |
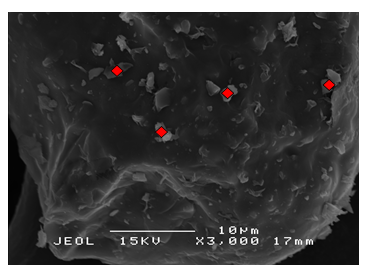 | Figure 2(b). SEM micrograph of 10 wt% K2CO3/SiO2 |
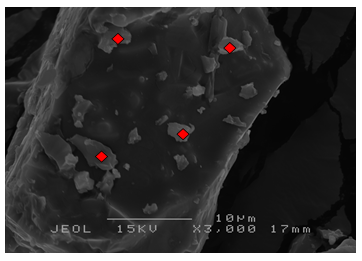 | Figure 2(c). SEM micrograph of 15 wt% K2CO3/SiO2 |
 | Figure 2(d). SEM micrograph of 20 wt% K2CO3/SiO2 |
3.1.4. Temperature Programmed Desorption of Carbon Dioxide (CO2-TPD)
- The basicity of the catalyst was evaluated using CO2-temperature programmed desorption (CO2-TPD). Figure 3 shows the CO2-TPD profiles of K2CO3/SiO2 catalysts. The CO2-TPD curves demonstrate the base strength of K2CO3-supported silica with different weight percentages. The quantification of desorbed CO2 is accomplished by calculating the area under the peak and the amount obtained for each sample (Table 4).
|
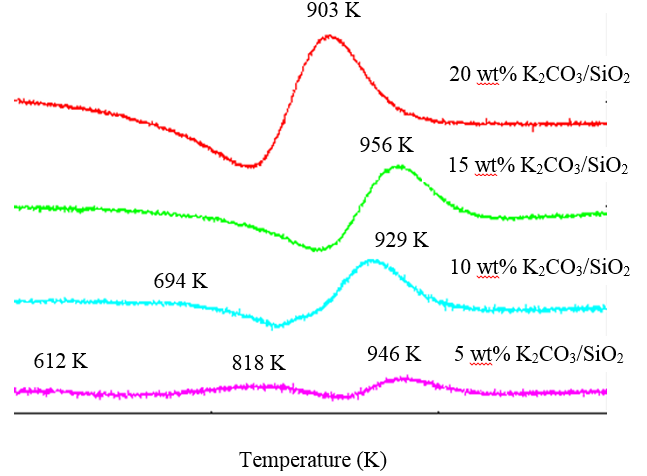 | Figure 3. CO2-TPD profiles of catalyst with different K2CO3 loading |
3.2. Analysis of Biodiesel
3.2.1. 1H-Nuclear Magnetic Resonance (1H-NMR)
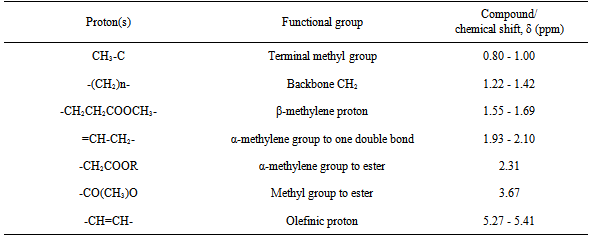
- 1H-Nuclear Magnetic Resonance (1H-NMR) was used to quantify fatty compounds in biodiesel based on the fact that the amplitude of a proton nuclear magnetic resonance (1H-NMR) signal is proportional to the number of hydrogen nuclei contained in the molecule [28]. Figure 4 shows the spectrum of the biodiesel obtained at optimum condition by using K2CO3/SiO2 catalyst. However, without using K2CO3/SiO2 catalyst, biodiesel cannot be produced. The signal for methylene protons appears at 2.3 ppm which together with ester group in triglycerides. After transesterification, the methoxy protons of methyl esters appear at 3.7 ppm (Figure 4 and Table 5), which describes the typical chemical structures related to the 1H-NMR spectrum.
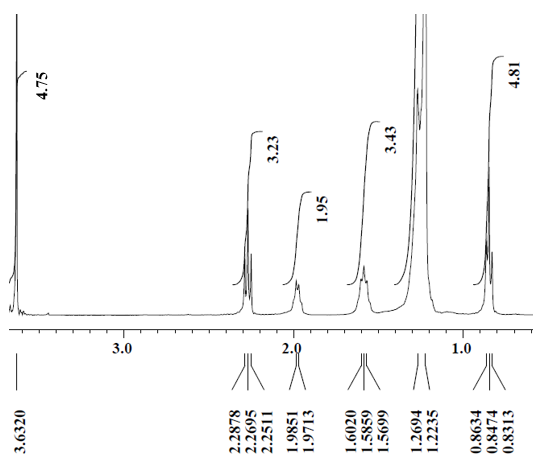 | Figure 4. 1H-NMR spectrum of biodiesel produced at reaction time 3 h, reaction temperature of 333 K, methanol to oil molar ratio of 20:1 with 20 wt% catalysts loading and 4wt% of catalyst amount |
|
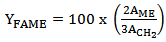 | (2) |
3.2.2. Component Determination in the Biodiesel by Gas Chromatography (GC)
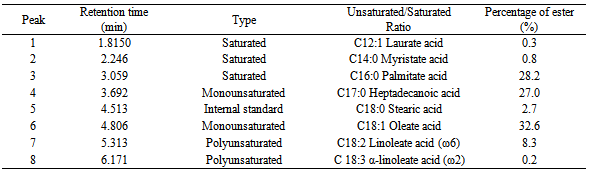
- The synthesized biodiesel products were analysed by gas chromatography to determine the composition of fatty acid methyl esters (Figure 5). Each peak corresponds to a fatty acid methyl ester component of palm oil and was identified using the library match software. The identities of the fatty acid methyl esters (FAME) were verified by comparing the respective retention time data with mass spectroscopic analysis.
|
4. Conclusions
- Silica loaded with K2CO3 catalyst was successfully prepared by the impregnation method and improved biodiesel production. The influence of the different K2CO3 loadings on silica, which was varied from 5, 10, 15, and 20 wt% were studied comprehensively. The addition of K2CO3 on SiO2 significantly influenced the crystallinity of the supported catalyst but also affected the particle size of the catalyst, which was found to decrease with increased amounts of K2CO3. Additionally, BET surface area analysis revealed that K2CO3-supported SiO2 has a small surface area but its high pore diameter allows it to accommodate bulky triglyceride molecules. According to the CO2-TPD results, the addition of K2CO3 on SiO2 results in the creation of strong basic sites which is needed as the basicity of the catalyst plays an important role in base-catalyzed biodiesel production which in this case 20wt % catalyst which has shown having the highest number of basic site which is determined as the prime contributor to the highest activity for the transesterification reaction as opposed to other loadings.The transesterification reaction of palm oil catalyzed by silica loaded with K2CO3 prepared in this study revealed the efficiency of the catalyst for the reaction. It was found that up to 98.10% yield of biodiesel was obtained when palm oil was transesterified with methanol in the presence of K2CO3/SiO2 catalysts. The existence of the biodiesel was confirmed by 1H-NMR analysis in which the distinct peaks of methyl esters methoxyl group at signal 3.67 ppm, α methylene groups of esters at signal 2.31 ppm and methyl ester compositions were successfully quantified.
ACKNOWLEDGEMENTS
- This research was supported by Graduate Research Fellowship (GRF). The authors gratefully acknowledge the contribution from Malaysian Palm Oil Board (MPOB) for helping with the yield analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML