-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2013; 3(6): 91-105
doi:10.5923/j.ep.20130306.01
Comparison among Various Emerging PV Cells with History, Current Status and Future Challenges
S. M. Shauddin
Institute of Radiation and Polymer Technology, Atomic Energy Research Establishment, Bangladesh Atomic Energy Commission, Savar, Dhaka, Bangladesh
Correspondence to: S. M. Shauddin, Institute of Radiation and Polymer Technology, Atomic Energy Research Establishment, Bangladesh Atomic Energy Commission, Savar, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Due to energy crises in the future, much effort is being directed towards alternate sources. Solar energy is accepted as a novel substitute for conventional sources of energy. A solar cell is an electrical device that converts the energy of light (solar energy) directly into electricity by the photovoltaic effect. This article, briefly describes about the evaluation of various emerging photovoltaic solar cells out of the long list of various types of solar cells available on the market. Since it is widely accepted that solar cells can be broadly categorized into one of three technology “generations” and the “third generation” usually reserved for so-called emerging technologies. Emerging PV is one of the fastest growing of all the renewable energy technologies in fact, it is one of the fastest growing industries at present. Because there is a strong need for the development of photovoltaic cells with low cost, high efficiency, and good stability. The article further discusses in detail the constructions and the operation principles of various emerging PV cells. It presents applications, latest conversion efficiencies and comparable information about strength and weakness of these. It reviews general approaches, challenges and solutions, as well as latest research progress and future prospects in the field of emerging photovoltaic solar cells.
Keywords: Emerging PV, DSSC, Quantum dot, BIPV
Cite this paper: S. M. Shauddin, Comparison among Various Emerging PV Cells with History, Current Status and Future Challenges, Energy and Power, Vol. 3 No. 6, 2013, pp. 91-105. doi: 10.5923/j.ep.20130306.01.
Article Outline
1. Introduction
- It is expected that the global energy demand will be doubled within the next 50 years. Fossil fuels, however, are running out and are held responsible for the increased concentration of carbon dioxide in the earth’s atmosphere. Hence, developing environmentally friendly, renewable energy is one of the challenges to society in the 21st century. Ever-increasing world energy demand, depleting non - renewable energy resources and disruptive climate change due to greenhouse gases has aroused much interest in alternative renewable energy sources. Solar energy is one of the best available alternatives, for it is both abundant and clean. All wind, fossil fuel, hydro and biomass energy have their origins in sunlight. Solar energy falls on the surface of the earth at a rate of 120 petawatts, (1 petawatt = 1015 watt). This means all the solar energy received from the sun in one days can satisfied the whole world’s demand for more than 20 years. A solar cell is an electrical device that converts the energy of light (solar energy) directly into electricity by the photovoltaic effect. The photovoltaic effect was discovered by Becquerel in 1839, but it was until the 1950s that a semiconductor device for converting sunlight energy into electrical energy was developed. Since then, such devices have evolved, and nowadays solar cells are made of different materials and structures. Although most of the semiconductor materials exhibit the photovoltaic effect, they should have a bandgap greater than 1.0 eV to be suitable for practical solar cells. Discovery of photovoltaic effect in silicon (Si) diode in 1954 dawned the era of modern solid state photovoltaic (PV) technology[1]. Since then Si solar cells have evolved as the most mature photovoltaic technology and represent over 90% of the present day photovoltaic market worldwide[2]. The PV market is mushrooming at a rate of 48% since 2002 and is the world’s fastest growing energy technology. In spite of this tremendous growth in PV sector, energy from PV technology accounts for less than 0.1% of the world energy demand. The primary reason for this is high module and installation costs of silicon photovoltaics. There is a strong need for the development of photovoltaic cells with low cost, high efficiency, and good stability. In the past decade a new class of photovoltaics based on organic materials has emerged and are a promising alternative to inorganic solar cells because of their low processing cost, ease of processibility, roll-to-roll processiblity for large area devices and mechanical flexibility[3,4]. The low efficiency and shorter lifetime of organic PVs is compensated by their low module cost, which provides a strong impetus to investigate organic photovoltaic (OPV) technology[5]. It is widely accepted that solar cells can be broadly categorized into one of three technology “generations.” First generation cells are crystalline. Second generation cells are amorphous thin films of silicon and other materials meant to reduce costs normally associated with conventional semiconductor wafer production. And “third generation” usually reserved for so-called emerging technologies. The first generation of solar cells, also known as silicon wafer-based photovoltaic, is the dominant technology for terrestrial applications today, accounting for more than 85% of the solar cell market. Single-crystalline and multi-crystalline wafers, used in commercial production, allow power conversion efficiencies up to 25%, although the fabrication technologies at present limit them to about 15 to 20%. The second generation of photovoltaic materials is based on the use of thin-film deposits of semiconductors, such as amorphous silicon, cadmium telluride, copper indium gallium diselenide or copper indium sulfide. The efficiencies of thin film solar cells tend to be lower compared to conventional solar cells, around 6% to 10%, but manufacturing costs are also lower, so that a price in terms of $/watt of electrical output can be reduced. Besides, decreased mass allows fitting panels on light materials or flexible materials, even textiles. Emerging PV is one of the fastest growing of all the renewable energy technologies, in fact, it is one of the fastest growing industries at present[1]. The third generation of photovoltaic cells is a research goal: a dramatic increase in efficiency that maintains the cost advantage of second-generation materials. The approaches include dye-sensitized nanocrystalline or Gratzel solar cells, organic polymer-based photovoltaics, tandem (or multi-junction) solar cells, hot carrier solar cells, multi-band and thermophotovoltaic solar cells.
2. Emerging Photovoltaic Cells
- A solar cell (also called a photovoltaic cell) is an electrical device that converts the energy of light directly into electricity by the photovoltaic effect. It is a form of photoelectric cell (in that its electrical characteristics—e.g. current, voltage, or resistance—vary when light is incident upon it) which, when exposed to light, can generate and support an electric current without being attached to any external voltage source. A dye-sensitized solar cell (DSSC, DSC or DYSC [6]) is a low-cost solar cell belonging to the group of thin film solar cells[7]. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a photoelectrochemical system. An organic solar cell or plastic solar cell is a type of polymer solar cell that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules,[8] for light absorption and charge transport to produce electricity from sunlight by the photovoltaic effect. Quantum dot solar cells are an emerging field in solar cell research that uses quantum dots as the absorbing photovoltaic material, as opposed to better-known bulk materials such as silicon, copper indium gallium selenide (CIGS) or CdTe. QD solarcells have been realized as photoelectrochemical cells and solid-state devices. Multi - junction solar cells or tandem cells are solar cells containing several p-n junctions. Each junction is tuned to a different wavelength of light, reducing one of the largest inherent sources of losses, and thereby increasing efficiency.
3. History of Emerging PV Cells
- The photovoltaic effect was first experimentally demonstrated by French physicist A. E. Becquerel. In 1839, at age 19, experimenting in his father's laboratory, he built the world's first photovoltaic cell. Willoughby Smith first described the "Effect of Light on Selenium during the passage of an Electric Current" in an article that was published in the 20 February 1873 issue of Nature. However, it was not until 1883 that the first solid state photovoltaic cell was built, by Charles Fritts, who coated the semiconductor selenium with an extremely thin layer of gold to form the junctions. The device was only around 1% efficient. In 1888 Russian physicist Aleksandr Stoletov built the first photoelectric cell based on the outer photoelectric effect discovered by Heinrich Hertz earlier in 1887[9]. The dye-sensitized solar cell (DSC or DSSC) grew out of studies of photosynthesis. In the late 1960s, while working with chlorophyll extracted from plants, researchers at Berkeley realized that organic dyes could produce electricity at the oxide electrode of an electrochemical cell. Increasing the surface area for charge collection was recognized as the path to improving cell efficiency. Eventually, a team at the Swiss École Polytechnique Fédérale de Lausanne identified titanium dioxide (TiO2) as the ideal anode material. This basic cell design is now fundamental to most DSSC research and is known as the Grätzell cell after its inventor. Cells with phthalocyanine as an organic layer were investigated at the early stage. As early as 1958, Kearns et al. reported the photovoltaic effect or the creation of voltage of a cell based on magnesium phthalocyanine a macrocyclic compound having an alternating nitrogen atom-carbon atom ring structure (MgPh), which had a photovoltage of 200 mV[10]. Ghosh et al. investigated the Al/MgPh/Ag cell, and obtained photovoltaic efficiency of 0.01% under illumination at 690nm[11]. The idea of using quantum dot solar cells was first noted by Burnham and Duggan in 1990[12]. At that stage, the science of quantum dots, or "wells" as they were known, was in its infant stage. Hadipour et al. demonstrated the first polymer tandem cell using two different polymer BHJ systems with complementary absorption range[13]. In this work both the bottom anode and the middle electrode were a semitransparent Au layer creating an optical cavity in the bottom cell. The optical cavity was tuned such that there is maximum overlap between the transmission through the bottom cell and the absorption of top low band gap cell. The reported efficiencies were low because the partially reflective metal layers reduce the amount of light absorbed by the sub-cells.
4. Constructions and Working Principles of Emerging PV Cells
- Various materials display varying efficiencies and have varying costs. Materials for efficient solar cells must have characteristics matched to the spectrum of available light. Some cells are designed to efficiently convert wavelengths of solar light that reach the Earth surface. However, some solar cells are optimized for light absorption beyond earth's atmosphere as well. Light absorbing materials can often be used in multiple physical configurations to take advantage of different light absorption and charge separation mechanisms. Many currently available solar cells are made from bulk materials that are cut into wafers between 180 to 240 micrometers thick that are then processed like other semiconductors. Other materials are made as thin-films layers, organic dyes, and organic polymers that are deposited on supporting substrates. A third group are made from nanocrystals and used as quantum dots (electron confined nanoparticles). Silicon remains the only material that is well-researched in both bulk and thin-film forms. The solar cell works in three steps:1) Photons in sunlight hit the solar panel and are absorbed by semiconducting materials, such as silicon.2) Electrons (negatively charged) are knocked loose from their atoms, causing an electric potential difference. Current starts flowing through the material to cancel the potential and this electricity is captured. Due to the special composition of solar cells, the electrons are only allowed to move in a single direction.3) An array of solar cells converts solar energy into a usable amount of direct current (DC) electricity.
4.1. Construction and Working Principle of DSSC
- In the case of the original Grätzel and O'Regan design, the cell has 3 primary parts illustrated in Fig-1 below. On top is a transparent anode made of fluoride-doped tin dioxide (SnO2:F) deposited on the back of a (typically glass) plate. On the back of this conductive plate is a thin layer of titanium dioxide (TiO2), which forms into a highly porous structure with an extremely high surface area. TiO2 only absorbs a small fraction of the solar photons (those in the UV)[14]. The plate is then immersed in a mixture of a photosensitive ruthenium-polypyridine dye (also called molecular sensitizers[14]) and a solvent. After soaking the film in the dye solution, a thin layer of the dye is left covalently bonded to the surface of the TiO2. A separate plate is then made with a thin layer of the iodide electrolyte spread over a conductive sheet, typically platinum metal. The two plates are then joined and sealed together to prevent the electrolyte from leaking. The construction is simple enough that there are hobby kits available to hand-construct them[15]. Although they use a number of "advanced" materials, these are inexpensive compared to the silicon needed for normal cells because they require no expensive manufacturing steps. TiO2, for instance, is already widely used as a paint base.
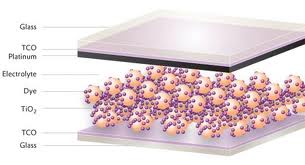 | Figure 1. Sketch of a dye sensitized solar cell |
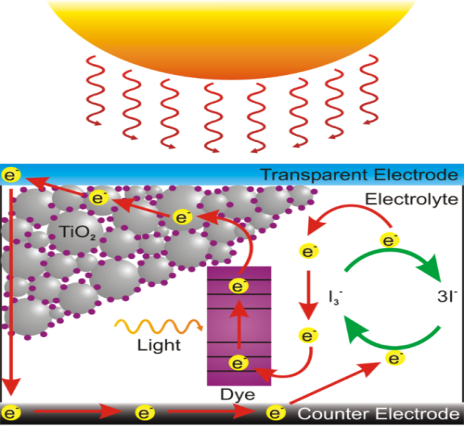 | Figure 2. The mechanism of DSSC |
4.2. Construction and Working Principle of OPVC
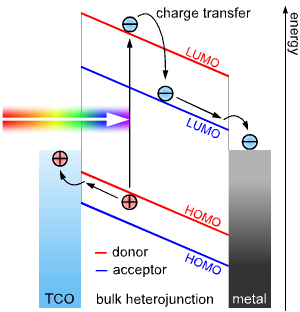
- Single layer organic photovoltaic cells are the simplest of the various forms of organic photovoltaic cells. These cells are made by sandwiching a layer of organic electronic materials between two metallic conductors, typically a layer of indium tin oxide (ITO) with high work function and a layer of low work function metal such as Al, Mg or Ca. The basic structure of such a cell is illustrated in Figure 3. The difference of work function between the two conductors sets up an electric field in the organic layer. When the organic layer absorbs light, electrons will be excited to the Lowest Unoccupied Molecular Orbital (LUMO) and leave holes in the Highest Occupied Molecular Orbital (HOMO) forming excitons. The potential created by the different work functions helps to separate the exciton pairs, pulling electrons to the positive electrode (an electrical conductor used to make contact with a non-metallic part of a circuit) and holes to the negative electrode. The current and voltage resulting from this process can be used to do work. Using electric fields is not the best way to break up excitons: heterojunction based cells which rely on effective fields are more effective[16-18].
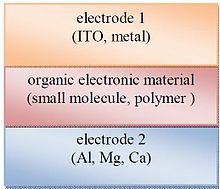 | Figure 3. Sketch of a single layer organic photovoltaic cell |
4.3. Construction and Working Principle of QDSC
- Quantum dot solar cells are made from cadmium-sulfide on a titanium dioxide substrate coated with organic molecules to interconnect the metal substrate and the quantum dots. Most three band gap layers include indium-gallium-arsenide, indium-gallium-phosphide, and germanium. Many chemical combinations are at work. The size of the nanoparticles used in the organic interconnect layer, seem to have more direct impact on the efficiency of quantum dot solar cells. The total thickness of a multi-junction solar cell has to be less than two nanometer, requiring an extremely fine level of precision. In a QDSC, a mesoporous layer of titanium dioxide nanoparticles forms the backbone of the cell, much like in a DSSC. This TiO2 layer can then be made photoactive by coating with semiconductor quantum dots using chemical bath deposition, electrophoretic deposition, or successive ionic layer adsorption and reaction. The electrical circuit is then completed through the use of a liquid or solid redox couple. The basic structure of such a cell is illustrated in Figure 5.
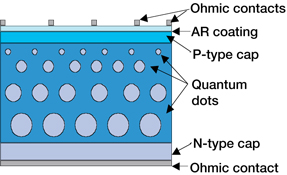 | Figure 5. Quantum-dot (QD)-enhanced solar-cell design concept |
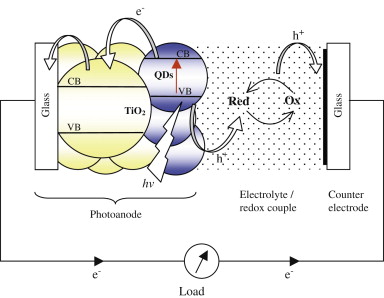 | Figure 6. Operating principle of a quantum dot solar cell |
- The common feature of QD-based solar cells is quantum confinement of the exciton in the absorber material leading to a size-dependent absorption spectrum. Here we review the working principle of the most common QD-based solar cell configurations such as QDSCs. QDSCs are based on a nanostructure of a wide-bandgap material that is sensitized with a QD monolayer. Conceptual similarities and differences to dye-sensitized solar cells (DSCs)[20] and extremely thin absorber (ETA) cells[21]. Nanostructured wide-bandgap semiconductor films provide a microscopic surface area, orders of magnitude larger than their geometric area, which can be sensitized with a thin absorber layer of low absorbance. The low optical density of a QD monolayer is compensated by a light path that passes through tens to hundreds of QD monolayers. Dye-sensitized solar cells and ETA cells make use of the same concept, and light to electric power conversion efficiencies above 10% have been reached with DSCs. In summary, the main working principle of QDSCs is very similar to DSCs, but further effects such as photoinduced bandedge movement can occur in QDSCs, depending on the composition of the cell material. The basic operating principle of such a cell is illustrated in Figure 6.
4.4. Construction and Working Principle of OTSC
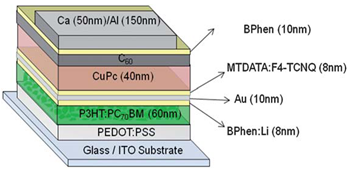
- The structure of an organic tandem photovoltaic (PV) cell is illustrated in figure 7. Indium tin oxide (ITO) coated glass is used as the anode. The donor (D) copper phthalocyanine (CuPc), acceptor (A) 3,4,9,10 – perylenetetracarboxylic - bis-benzimidazole (PTCBI), and a Ag cathode are deposited by thermal evaporation. The tandem cell is a series connection of two organic PV cells which allows for an increase in open circuit voltage to twice that of a single device. Upon light absorption, excitons are formed in both photovoltaic subcells. After dissociation at a DA interface, the hole in the front PV cell and electron in the back PV cell are collected at the adjacent electrodes. To prevent build-up of charge within the cells, the electron in the front PV cell and hole in the back PV cell diffuse to the metal nanocluster layer where they recombine. The advantage of this structure is that individual devices can be made thin to allow for a large percentage of excitons to contribute to photocurrent, while the device itself is thick to allow for a large absorption efficiency. This tandem device structure, however, led to an increase in power efficiency which was more than twice that of a single device, from 1.1% for a single DA heterojunction device to 2.5% for the tandem device.
5. Applications of Emerging PV Cells
- To make practical use of the solar-generated energy, the electricity is most often fed into the electricity grid using inverters (grid-connected photovoltaic systems); in stand-alone systems, batteries are used to store the energy that is not needed immediately. There are several applications for solar energy, for instance: electricity generation, photochemical, solar propulsion, solar desalination, and room temperate control. The collection of solar energy and its transfer to electricity energy will have wide application and deep impact on our society, so it has attracted the attention of the researchers. Solar panels can be used to power or recharge portable devices. The present situation indicates that organic solar cells cannot substitute for silicon cells in the energy conversion field. However their use seems to be more targeted towards specific applications such as recharging surfaces for laptops, personal mobile phones, clothes, and packages, or to supply the power for small portable devices, such as cellphones and MP3 players, small home electronics and mobile electronics attachment, BIPV such as building’s exterior wall, window, or blinder, and power generation. In an ideal situation, quantum dot solar cell could be built on flexible polymer materials so that it can be used as coating for portable electronics. It should also be capable of being weaved into synthetic fabrics for clothing and car upholstery. In near future, quantum dot solar cells can be seen to have widespread applications in electricity generation, supplementing or supplanting the need for fossil fuel use for lighting, telecommunications, and transportation.
6. Strengths and Weaknesses of Emerging PV Cells
- The major strength of the solar cell is that it can provide a sustainable, renewable and environmentally friendly source of energy. This positions solar energy to differentiate itself from traditional, carbon-based energy sources. The major weakness of the solar cell is that its product is costly to implement and is difficult to produce high levels of solar power. Solar energy can have difficulty competing with traditional forms of energy on the basis of price.
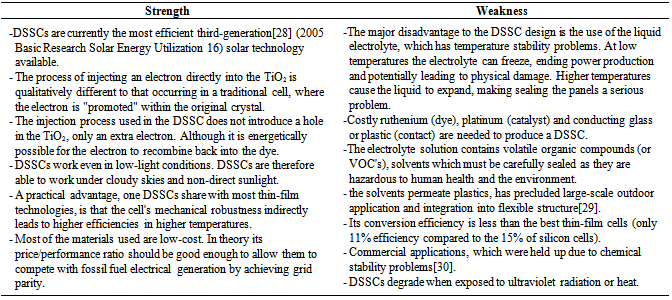
6.1. Strength and Weakness of DSSC
- The following table 1 represents the strength and weakness of dye sensitized cell:
|
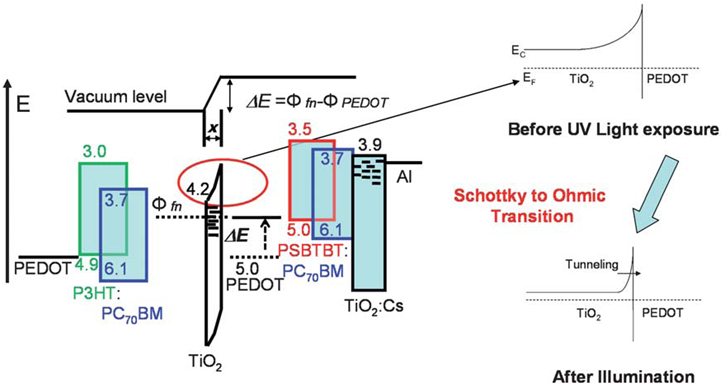 | Figure 8. Energy band diagram of a tandem cell and the schottky to Ohmic transition at the interface between TiO2 and PEDOT upon irradiating with UV light |
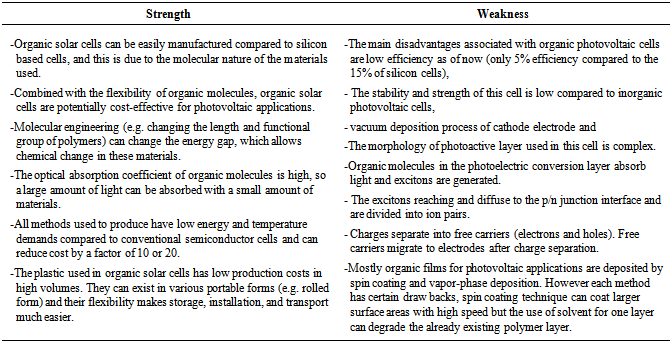
6.2. Strength and Weakness of OPVC
- The following table 2 represents the strength and weakness of organic photovoltaic cell:
|
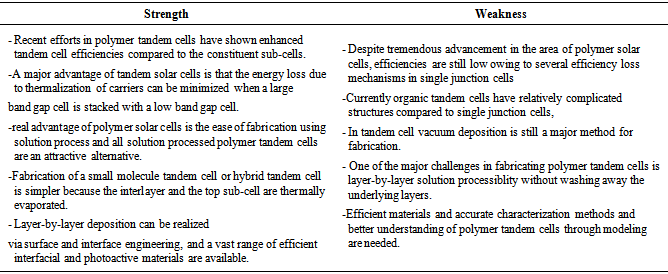
6.3. Strength and Weakness of QDSC
- The following table 3 represents the strength and weakness of quantum dot cell:
|
6.4. Strength and Weakness of OTSC
- The following table 4 represents the strength and weakness of organic tandem cell:
|
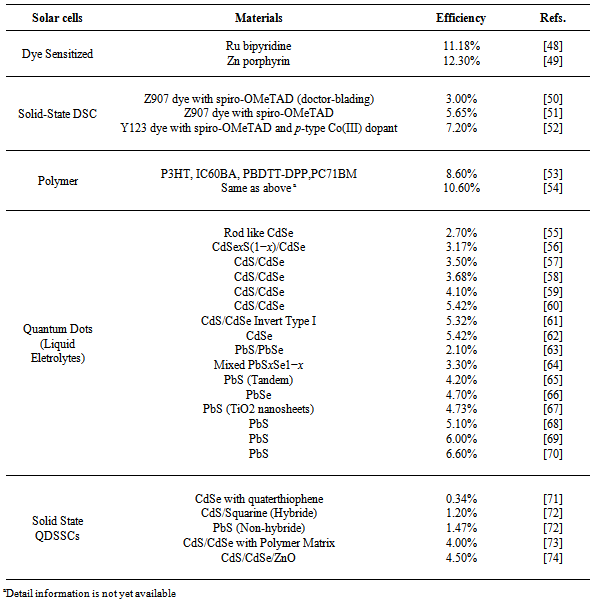
7. Efficiencies of Emerging PV Cells
- The most important parameter of any solar cell is its efficiency, that is, how much of the sunlight energy is converted into electrical energy. Shockley and Queisser established that a single-junction solar cell can reach a maximum efficiency of 30%[36]. Solar cell efficiency is the ratio of the electrical output of a solar cell to the incident energy in the form of sunlight. The energy conversion efficiency (η) of a solar cell is the percentage of the solar energy to which the cell is exposed that is converted into electrical energy. This is calculated by dividing a cell's power output (in watts) at its maximum power point (Pm) by the input light (E, in W/m2) and the surface area of the solar cell (Ac in m2). By convention, solar cell efficiencies are measured under standard test conditions (STC) unless stated otherwise. STC specifies a temperature of 25°C and an irradiance of 1000 W/m2 with an air mass 1.5 (AM1.5) spectrum. These conditions correspond to a clear day with sunlight incident upon a sun-facing 37°-tilted surface with the sun at an angle of 41.81° above the horizon[37,38]. The current generated by a DSSC, for comparison, a traditional silicon-based solar cell offers about 35 mA/cm2, whereas current DSSCs offer about 20 mA/cm2. Overall peak power conversion efficiency for current DSSCs is about 11%[39,40]. Current record for prototypes lies at 12.3%[41]. Energy conversion efficiencies achieved to date using conductive polymers are low compared to inorganic materials. However, it has improved quickly in the last few years and the highest NREL (National Renewable Energy Laboratory) certified efficiency has reached 8.3% for the Konarka Power Plastic[42]. In addition, these cells could be beneficial for some applications where mechanical flexibility and disposability are important. Quantum dot solar cells result in an efficiency of 7.0%[43] which is among the highest observed for QDSCs and, although quite low compared to that of commercial bulk silicon cells (about 17%), it has a potential for improvement beyond silicon cells by means of hot carrier extraction and efficient multiple exciton generation. Traditional single-junction Organic tandem photovoltaic (PV) cells have a maximum theoretical efficiency of 34%, a theoretical "infinite-junction" cell would improve this to 87% under highly concentrated sunlight. The best efficiencies for tandem cells that have been reported so far are slightly over 7% because of several issues and challenges concerning polymer tandem cells [44-46]. Every year NREL publishes the Best Research-Cell efficiencies data (Figure 9). It contains information about the time evolution of solar cells made of several materials and structures.
|
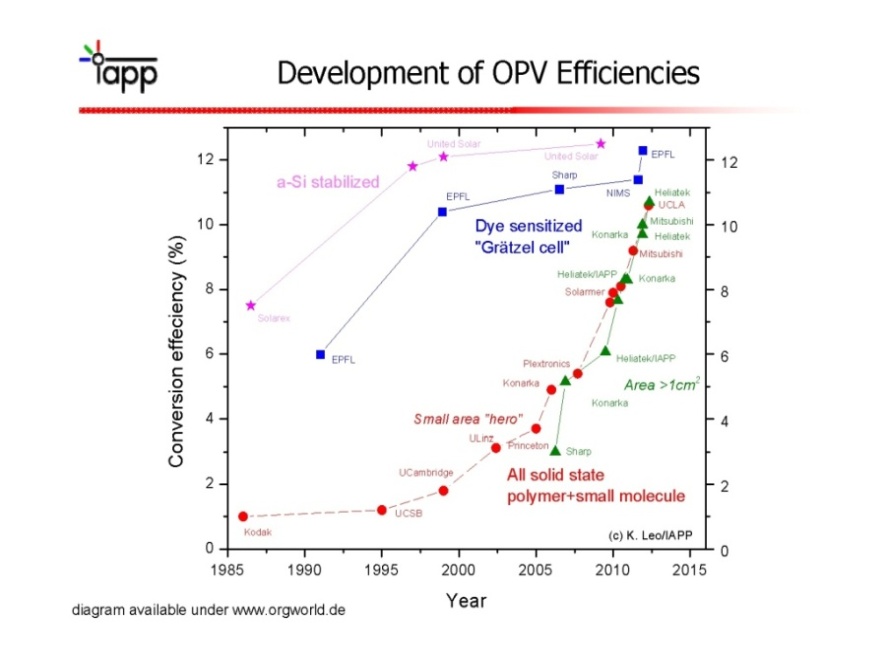 | Figure 9. NREL's data about record of solar cells efficiencies[47] |
8. Present Status and Future Prospects of Emerging PV Cells
- A number of PV technologies—frequently referred to as third-generation PV—are being developed. Dye-sensitized solar cells use dye molecules absorbed onto a nanostructured substrate and immersed in a liquid or gel electrolyte to absorb solar radiation and have demonstrated laboratory efficiencies as high as 11.1%. In 2011 Dyesol and Tata Steel Europe announced in June the development of the world´s largest dye sensitized photovoltaic module, printed onto steel in a continuous line[75]. In 2012 Northwestern University researchers announced[76] a solution to a primary problem of DSSCs, that of difficulties in using and containing the liquid electrolyte and the consequent relatively short useful life of the device. This is achieved through the use of nanotechnology and the conversion of the liquid electrolyte to a solid. The current efficiency is about half that of silicon cells, but the cells are lightweight and potentially of much lower cost to produce. Organic PV (OPV) solar cells, based on polymers or small molecules with semiconductor properties, have demonstrated laboratory cell efficiencies above 8%; organic modules have the potential for low-cost manufacturing using existing printing and lamination technologies (Shaheen et al. 2005). Polymer solar cells currently suffer from a lack of enough efficiency for large scale applications and stability problems[77] but their promise of extremely cheap production[78] and eventually high efficiency values[79] has led them to be one of the most popular fields in solar cell research. It is worth mentioning that state-of-the-art devices produced in academic labs – with the record currently held by Yang Yang’s group in UCLA – have reached certified efficiencies above 8%[80] while devices produced which have remained unpublished – probably to maintain secrecy for industrial applications – are known to have already gone above 10%[81]. There are significant challenges to the commercialization of solution-processed organic solar cells, dye-sensitized cells with certain electrolytes, and quantum dots due to the stability of the materials against oxygen and water ingress. This limits the lifetime of these devices to anywhere from a few hundred hours to 2 years. This issue is being addressed through efforts to develop improved, yet cost-effective, encapsulants. In addition, organic and dye-sensitized solar cells use dyes that have been shown to degrade when put in direct sunlight for long periods of time, a significant issue to have in a solar cell. Further research and development (R&D) is needed to improve the viability of these materials. Difficulties associated with organic photovoltaic cells include their low quantum efficiency (~3%) in comparison with inorganic photovoltaic devices; due largely to the large band gap of organic materials. Instabilities against oxidation and reduction, recrystallization and temperature variations can also lead to device degradation and decreased performance over time. This occurs to different extents for devices with different compositions, and is an area into which active research is taking place[82]. Other important factors include the exciton diffusion length; charge separation and charge collection; and charge transport and mobility, which are affected by the presence of impurities. Due to its small size, Quantum dot nanocrystals are more suitable for the production of energy-efficient solar cells. They have bandgaps that are easily tunable across a wide range of energy levels (solar spectrum). Detailed research is on regarding the potential performance of the quantum dot approach. Earlier, costly molecular beam epitaxy process was used to produce quantum dot solar cells. Nowadays, alternative inexpensive fabrication methods have been developed. These research attempts depend on quantum dot synthesis using wet chemistry (colloidal quantum dots – CQDs). Nowadays, advanced Quantum dot solar cells are used for successive ionic layer absorption and reaction (SILAR) for semiconductor deposition. The efficiency rate is 5.4% which is highest observed for QDSCs. However, the performance figure is quite low compared to that of commercial bulk silicon cells (about 17%). Still, quantum dot solar cells have the potential for improvement beyond silicon cells. Quantum dots—nanospheres with physical properties similar to both bulk semiconductors and discrete molecules—have the potential to achieve higher efficiencies through multiple exciton generation, but they have not yet been used to produce efficient PV cells. Although research is still at a pre-commercialization stage, in the future quantum dot based photovoltaics may offer advantages such as mechanical flexibility (as in quantum dot-polymer composite photovoltaics[83] as well as low cost, clean power generation[84] and an efficiency of 65%[85]. The interface properties within the device are critical for its operation, and a fundamental understanding of how to control and engineer their properties is lacking. Research in this direction using interface dipoles and coating layers has just started, and further progress is expected in the near future. New methods for energy-level alignment should boost the QDSC efficiency to compete with the conversion efficiency of 15–20%, typical for polycrystalline silicon, but at a significant lower cost. Enhancing photon absorption from a given amount of colloidal quantum dot film offers significant prospects for increasing the available photocurrent. The electronic properties of colloidal quantum dot films currently limit device performance. To exceed power-conversion efficiencies of 10% in a single-junction planar cell, a material’s electron and hole mobility should exceed 10–1 cm2 V-1 s-1 and its bandgap should be as trap-free as possible (<1014 cm–3 deep traps)[86]. Much progress has been made towards this objective through advances in the packing and passivation of colloidal quantum dots in thin solid films[87-90]. Quantum dot solar cells are also made from cadmium-sulfide on a titanium dioxide substrate coated with organic molecules to interconnect the metal substrate and the quantum dots. Most three band gap layers include indium-gallium-arsenide, indium-gallium-phosphide, and germanium. Many chemical combinations are at work. The size of the nanoparticles used in the organic interconnect layer, seem to have more direct impact on the efficiency of quantum dot solar cells. The total thickness of a multi-junction solar cell has to be less than two nanometer, requiring an extremely fine level of precision. Research into quantum dot solar cells, aim to make solar cells both more efficient and less costly to manufacture. In an ideal situation, quantum dot solar cell could be built on flexible polymer materials so that it can be used as coating for portable electronics. It should also be capable of being weaved into synthetic fabrics for clothing and car upholstery. Polymer tandem solar cells, where two or more single junction cells that absorb in different wavelength range are connected in tandem, are being explored to overcome the limitations of single junction cells. It was not until recently that progress in polymer tandem cells was retarded because of several challenges and issues such as layer-by-layer solution processiblity, optimization of component cells and accurate characterization methods. Recent years have seen tremendous progress in polymer tandem cells owing to the development of efficient materials and accurate characterization methods and better understanding of polymer tandem cells through modeling. With the recent breakthroughs in organic/polymericphotovoltaicresearch, [91,92] it is believed that 10% conversion efficiency can be reached for single cells in the near future. The next target for the whole field would be at least 15%. Recent efforts in polymer tandem cells have shown enhanced tandem cell efficiencies compared to the constituent sub-cells. Layer-by-layer deposition can be realized via surface and interface engineering, and a vast range of efficient interfacial and photoactive materials are available. Various new approaches and device design have been proposed to enhance tandem cell performance, offering opportunity to incorporate newly synthesized low bandgap materials to achieve breakthrough efficiencies. Currently organic tandem cells have relatively complicated structures compared to single junction cells, raising concerns about processibility issues. For example, vacuum deposition is still a major method for fabrication, though all solution processed structures are highly desirable. The compromise between simple process/structures and high efficiency[93] has to be reached with respect to future applications. The new materials that solar energy can be harnessed with is one of the most exciting elements of the new technology. The flexible and lightweight physical characteristics of the different types of third generation solar cells makes many new applications possible. There is the possibility that solar cells could be integrated into clothing which would allow us to have personal wireless power without batteries. Another plausible application could be a type of automobile paint that is blended with polymer solar cells. This could help maintain the lightweight form of a solar car while still providing ample energy to power it.
9. Conclusions
- Solar energy will be widely utilized in industry, agriculture and daily life. Photovoltaic (PV) systems have recently attracted much attention due to their inherent advantages. Firstly, PV systems are capable of directly translating sunlight into electrical energy. The theoretical conversion efficiency of PV systems is relatively higher than other power generators. Secondly, PV systems do not necessarily contain movable parts. System wear induced by mechanical movement is avoided. Therefore, PV systems can work continuously free from maintenance longer than other power generation technologies. Many of the emerging cell types such as polymer and dye-sensitized cells, tandem cells built from stacks of conventional materials (such as amorphous silicon or gallium arsenide), and nanotechnology offer distinct advantages over traditional silicon photovoltaic, especially for applications in building integrated photovoltaics (BIPV) and uses where substrate flexibility is important. However, there are still issues that need to be addressed. For instance, DSC cells tend to have short lifetimes and other reliability concerns compared to the competition. And although DSSC leads the way for third generation technology with top cell efficiencies of over 11 percent, pushing it into the useful range for commercialization, progress has been relatively flat since the late 1990s. Fortunately, there are other nanostructure devices that have emerged more recently and have shown very rapid improvement in their short life. To encourage and to promote the direct utilization of solar energy, developed countries have been legislating and deploying solar initiatives[94-96]. Joint Research Centre (Europe) predicted that energy directly harvested from sunlight would be 20% of total energy consumption in 2050, and this value could be over 50% in 2100[97]. In this article I selected four of the most commonly studied and discussed emerging PV solar cells and reviewed their structure, performance, advantages and drawbacks. This report is intended to provide a brief summary for those who are interested in emerging PV technology based solar cells and as a reference for those who want to invest or work in this field.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML