-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2013; 3(4): 67-78
doi:10.5923/j.ep.20130304.05

Identification of Non-edible Seeds as Potential Feedstock for the Production and Application of Bio-diesel
Nikul K Patel 1, Padamanabhi S Nagar 2, Shailesh N Shah 3, Anand K Patel 4
1Department of Mechanical Engineering, The M S University of Baroda, Vadodara, 390001, India
2Department of Botany, The M S University of Baroda, Vadodara, 390001, India
3Department of Applied Chemistry, The M S University of Baroda, Vadodara, 390001, India
4Mechancial Engineering Department, LDRP Institute of Technology & Research, Gandhinagar, India
Correspondence to: Shailesh N Shah , Department of Applied Chemistry, The M S University of Baroda, Vadodara, 390001, India.
| Email: |  |
Copyright © 2013 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Increasing demand of conventional source of fuel has made people to consider on alternative fuels that can substitute the present demand by some percentage. This paper presents the state of the art in this area by reviewing number of contributions from the all three disciplines i.e. Botany, Chemistry and Engineering are taken together to critically review the work done in this field of Bioenergy. Large number of survey and work is done in identification of non-edible seeds as potential feed stock from which oil can be extracted. Experimental investigation has been done by several researchers in production of Bio-diesel from oil extracted, trans-esterified and studied different properties of fuel produced. This fuel and it’s different blends with diesel are used by engineers to understand the performance of engine. Number of experimental work is done using fuel available from non-edible oils feedstock to investigate performance of this biodiesel using compression ignition engine. In this review, the findings reported by different researchers have been summarized to portrait the use of non-edible seeds for the production, mathematical model and application of bio-diesel.
Keywords: Non-edible Seeds, Trans Esterification, Bio-diesel, Fuel Properties, Performance of Engine
Cite this paper: Nikul K Patel , Padamanabhi S Nagar , Shailesh N Shah , Anand K Patel , Identification of Non-edible Seeds as Potential Feedstock for the Production and Application of Bio-diesel, Energy and Power, Vol. 3 No. 4, 2013, pp. 67-78. doi: 10.5923/j.ep.20130304.05.
Article Outline
1. Introduction
- For the industrial and economic growth, there should be enough supply of electricity and transport facility in the country. The fossil fuels used are non-renewable and due to continue use, they will deplete in the future and there will be energy crisis in the world. The use of fossil fuels also pollutes the environment and causes environmental problems like global warming, greenhouse effect, air pollution etc. Moreover, important fossil fuels like oil and gas are concentrate in some of the countries, resulting the remaining countries like India have to completely depends on such countries which are producing oil and gas.. Due to Globalization, developing countries like India and China, the consumption of petroleum products and natural gas increases year by year at unbelievable rate. In India oil provides energy for 95% of transportation for which domestic supply of crude will satisfy only about 22% of the demand and the rest will have to be met from imported crude[1]. As a result, the import bill also increases. This adversely affects the economy of country. The cost of the diesel fuel increases due to increase in crude oil price which necessitate taking appropriate policy decisions in the country to fulfil future demand. Therefore, biodiesel is being considered to be alternative fuel to the diesel in the country[2]. In view of energy and environmental problems associated with the use of fossil fuels in power generation and for transportation, an increasing attention is being paid worldwide by the scientists and engineers alike for the utilization of renewable energy sources. Considering the demand of fossil fuels and the effect on climate, it is high time to think about alternate source of energy which can not only substitute the conventional energy sources but also keep the environment pollution free and long term solution. There are various types of renewable energy sources such as solar, wind, hydropower, ocean thermal energy, geothermal energy, wave energy, biomass and bio energy etc. It is very necessary to develop the source of renewable energy and increase its utilization to reduce dependency on fossil fuels and environmental pollution issues. These renewable energy sources will be very important in the future when fossil fuels are depleted. The biomass and bio-energy is one of the important renewable energy sources. Biodiesel may be produced from various sources such as vegetable oil/plant oil both edible as well as non-edible oils. Production of Bio-diesel from edible oil crops is not desirable as there are many concerns regarding the use of food crops as feedstock for fuel production and has created famous debates about food v/s fuel[1]. The high price of biodiesel derived from food grade vegetable oils makes it non-viable to compete economically with fossil - based diesel. Less expensive, non-edible vegetable oil / plant oil as potential feedstock for biodiesel production[3], is one of the key re-source of the renewable bioenergy.
2. Identification of Non-edible Seeds
- A fuel produced from natural, renewable sources such as vegetable oil, seeds and fats is the best alternative to present source of energy produced from fossil resource. Initially, the most commonly used oils for the production of Bio-diesel were soya bean, sunflower, palm, rapeseed, canola, cottonseed and jatropha[4]. Use of such edible oil for the production of Bio-diesel is unfeasible in India because of a vast gap between demand and supply of such oils. Thus in India only those oils can be utilized for the production of Bio-diesel which comes under the class of the non-edible seeds, which might not compete with edible. An additional necessity of such non-edible seeds is that it must be able to cultivate it on large scale on non-cropped marginal lands and waste lands. There is a long list of trees, shrubs and herbs available plentifully in India, which can be used for extracting oil and producing bio-diesel as fuel from them. There are many plants in India; however there are 77 non-edible Indian plants, which contain 30% or more oil in their seed, fruit or nut[5]. Table 1 show the list of botanical name of 77 species which are identified as potential non-edible seeds, fruit or nut can be explored for production of Bio-diesel in India.After its earlier plans to begin mandatory blending of bio-diesel with fossil diesel by the year 2005 failed to take off due to inadequate production of biodiesel caused by near complete reliance on one species, Jatropha carcass, India decided to broad base its feedstock and land choices in order to achieve 17% blending by year 2017[6]. Among all other alternative towards diesel substitution in Indian automobile sector as compared to Compressed Natural Gas (CNG), Liquefied Natural Gas (LNG), Compressed Natural Gas in combination with Hydrogen etc. Biodiesel based on non-edible oil is best[7], due to following reasons:• Non-edible oil species, which can grow on wasteland.
|
3. Production of Oil and its Methyl Esters from Non-edible Seeds
- Oil extracted from non-edible seeds cannot be directly used as fuel in automobile engine due to higher viscosities of oils. Moreover problems like injector coking, more engine deposits, ring sticking and thickening of engine lubricant are observed using straight vegetable oil as fuel. There are many ways and procedures to convert seed oil in diesel like fuel but trans-esterification is the one of the best process for production of biodiesel.
|
3.1. Jatropha Curcas L. (Ratanjyot)
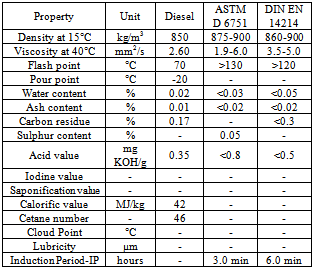
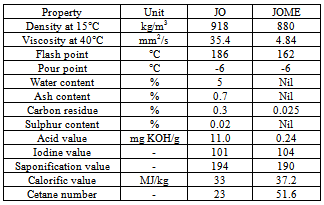
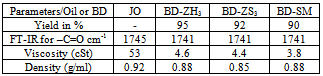
- Jatropha curcas L., non-edible seed from which oil (JO) was extracted and its methyl ester (JOME-biodiesel) was produced from it had given a yield of 97%[8]. Jatropha curcas L.., tree or shrub grows practically all over India under a variety of agro-climatic conditions and it is commonly found in most of the tropical and subtropical regions of the world. Free Fatty Acid (FFA) shows 16 components such as Oleic, Linoleic, Palmitic, Stearic, Palmitoliec, Linolenic, Arachidic, Margaric, Myristic, Caproic, Caprylic, Lauric, Capric, Saturated,Monounsaturated, and Polyunsaturated. Esterification was performed using acid catalyst (5% H2SO4) and methanol (20% of oil), while trans-esterification reaction was carried out for 2 hours keeping molar ratio of methanol to oil at 6:1 and sodium hydroxide concentration of 0.7 weight percentage of oil. Kinematic viscosity and cetane values are slightly higher than diesel, which is favorable for combustion. Higher flash point is advantageous for fuel transportation. It was observed that the calorific value of JOME is 37.2 MJ/kg, which is low compared to diesel fuel of 42 MJ/kg. JOME has higher cloud and pour point than conventional diesel which helps them working in cold environment. The various properties were found to be comparable with diesel and Table 3 shows the fuel properties of JO and JOME. When compared with various standards as per table 2 are in well accordance with them.
|
|
3.2. Simmondsia Chinesis L. (Jojoba)
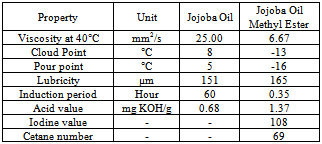
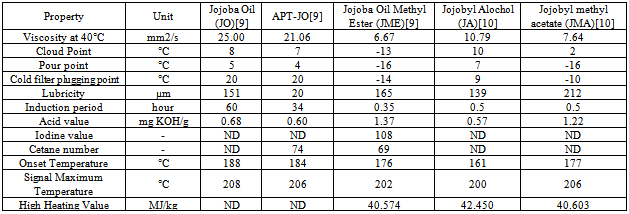
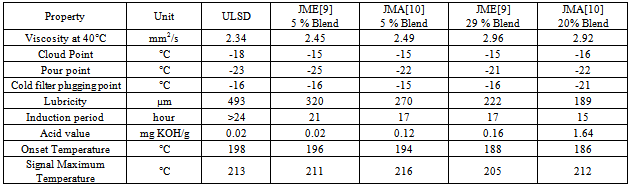
- The jojoba plant seeds contain 50 to 60 % by weight of non-edible oil which is suitable for production of biodiesel[10]. It is a perennial shrub belonging to Simmondsiaceae family that is native to the Mojave and Sonoran deserts of Mexico, California, Arizona and western part of India. Fourier transform infrared spectroscopy (FTIR) spectra were recorded on a Thermo Nicolet Nexus 470 FTIR system with a Smart ARK accessory containing a 45 ZeSe trough in a scanning range of 650 – 4000 cm-1 for 32 scans at a spectral resolution of 4 cm-1. The fatty acid profiles of jojoba oil and its methyl ester are Myristic, Palmitic, Stearic, Arachidic, Behenic, Palmitoleic, Oleic, Vaccenic, Gondoic, Erucic, Nervonic, Linoleic, Linolenic, Arachidonic and Docotetrasenoic. Acid – catalyzed pretreatment of jojoba oil was carried out as higher acid value and base catalyst lead to soap formations and consequently, increase in the formation of emulsions, which may impact on the yield of biodiesel. Transesterification of pretreated oil was done by adding freshly cut sodium metal into methyl alcohol, after which this mixture was heated at 65°C for 4 hour with stirring at reflux. Table 5 shows the properties of jojoba oil and its methyl ester which has the potential as an alternative nonfood feedstock for biodiesel production and different properties are is acceptable range when compared with standards describe in table 2. Viscosity is high as compare to diesel which may affect the flow of fuel. Lower pour point may help in using it in cold climatic conditions. Its lubricity property may be advantageous in using it as good lubricant.
|
|
|
3.3. Pongamia Pinnata (L.) Pierre (Karanja)
- Karanja seed kernels contain 27-39% of oil which can be used for production of its methyl ester. Karanja is a tree grown in parts of India and Australia. The potential availability of the oil is estimated to be 55,000 tons per year[12]. Fatty acid profile for karnaja oil and its methyl ester are Palmitoliec, Stearic, Oleic, linoleic, linolenic, arachidic, gondoic, erucic, and nervonic. Different methods used for the production of biodiesel from Karanja are direct use, micro emulsion, pyrolysis and trans esterification. Acid catalyzed transesterification uses Bronsted acid but H2SO4 is commonly used. Base catalyzed transesterification uses alkaline metal alkoxides give higher yield in short reaction time as compare to acid catalyst. The calorific value of biodiesel obtained from Karanja is 39656 kJ/kg, as compared to calorific value of conventional diesel which is 43405 kJ/kg. This reflects that heat content of the biodiesel obtained from Karanja oil is nearly 90% that of biodiesel.
3.4. Schlichera Oleosa Merr. (Kusum)
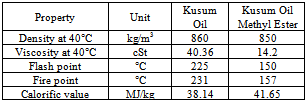
- Schlichera Oliosa seed kernels contain 40.3% of yellowish brown colored oil[13]. Kusum is widely available in sub-Himalayan region, Chattishgarh, throughout central and southern India, Burma, Ceylon, Java and Timor. FFA composition shows 16 components such as Myristic acid, Palmitic acid, Palmitoleic acid, Oleic acid, Linolelaidic acid, Cis Linoleic acid, alpha-Linolenic acid, Eicosenoic acid, Eicosadienoic acid, Heneicosanoic acid, Behenic acid, Erucic acid, Lignoceric acid and Docosahexaenoic acid. Alkaline-catalyzed esterification process could not produce biodiesel from high FFA oils from seeds of Kusum. A two-step esterification method is developed to produce biodiesel from Kusum from high FFA vegetable oil. The acid-catalyzed pretreatment process reduces the high FFA content of the crude oil to about 2% FFA. In second stage oil is transesterified using alkaline catalyst. The fatty acid composition was investigated by GC/MS after methylation. The flash point of biodiesel is higher than that of diesel but viscosity and density are found to be close to that of diesel. The performance of the biodiesel can be improved if the concentration of esters in biodiesel is lower. Table 8 shows important properties of unrefined Schlichera oleosa oil and its methyl esters when compared with standard as per table 3.1 are in well accordance with them.
|
3.5. Azadirachta Indica A. Juss. (Neem)
|
3.6. Calophyllum Inophyllum L. (Punnakka)
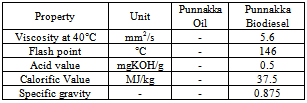
- Punnakka oil is produced from the tree called punna which belongs to the family clusiaceae and the tree is medium to large size with shining leaves and golden seeds. Punnakka oil is obtained by crushing the seeds and kernels are separated for drying in sun, the kernels of the seeds have high oil content of 75%[15]. This tree is found in many countries especially in coastal areas. Free Fatty Acid profile for Punnakka oil contains Palmatic acid, Hydnocarpic acid, Stearic acid, Oleic acid, Linoleic acid, Linolenic acid and Lignocerate acid. The optimum condition for esterification process was obtained and found that methanol to oil ratio of 0.65 v/v, acid catalyst concentration of 0.75% v/v of oil, reaction temperature of 60°C, reaction time of 60 minutes and settling time of 90 minutes. This was the first stage and in second stage catalyst concentration is for optimum condition above which the conversion efficiency is reduced. This will help in production of low cost punnakka oil for biodiesel production. Table 10 shows the various properties of Punnakka oil.
|
3.7. Ceiba Pentadra Gaertn. (Kapok)
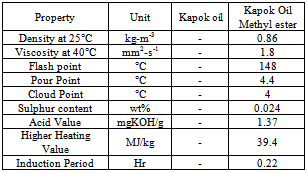
- The seeds contain 22-25% wt/wt oil contents. Kapok is a tree in a family of Malvaceae and locally known as Kekabu in America, West Africa and some part of India. It produces between 500 to 4000 fruits in one time, with each fruit containing 200 seeds. Kapok seed oil methyl esters were analyzed for fatty acid profile using Gas Chromatography Mass Spectroscopy. Palmatic acid, Capric acid, Linoleic acid, Oleic acid, Stearic acid, Sterculic acid and Arachidic acid were observed during the test. The pretreatment of kapok oil is done by acid esterification until the acid value is less than 2 mg KOH/g of oil or below 1% of FFA content before preceded to transesterification reaction[16]. The fuel properties were in well accordance with standards as compared with table 2 but further research and development is required to improve its fuel properties. Table 11 enumerates the fuel properties of Kapok seeds oil. The low temperature properties of Kapok Oil methyl ester can be improved by using different type of additives. The induction period that is oxidation stability was determined to be 12.3 hour which was beyond the standard values.
|
3.8. Sapindus Mukorossi Gaertp. (Soapnut)
- Soapnut seed kernels oil content is in range of 50-55% of seed weight which is identified as non-edible oil[17]. It grows wild from Afghanistan to China, ranging in altitudes from 200 to 1500 m also in India. Fatty acid methyl ester was characterized using gas chromatography with flame ionization detection using 50% cyanopropyl polysiloxane phase. The fatty acid of biodiesel produced from soapnut oil obtained by gas chromatography are palmitic acid, patmitoleic acid, stearic acid, oleic acid, linoleic acid, alpha or gamma linolenic acid, arachidic acid, eicosenic acid, behenic acid, erucic acid, Lignoceric acid and others. Acid catalyzed transesterification with H2SO4 in methanol was used to produce its fatty acid methyl ester.
3.9. Moringa Oleifera Lam. (Drum Stick)
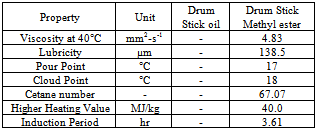
- Drum stick seeds contain between 33 and 41% w/w of vegetable oil. They are most widely known and are indigenous to sub-Himalayan regions of northwest India, Africa, Arabia, South east Asia, the Pacific and Caribbean Islands and South America. Thus they are available in large content.[18]. The fatty ester profile of the drum stick oil as determined by gas chromatography gives Palmitoliec, Stearic, oleic, linoleic, linolenic, arachidic, gondoic, erucic, nervonic, behenic acid, and polyunsaturated fatty acid. Drumstick oil was pre-treated with acid to reduce its acid value to 0.953. After acid pre-treatment biodiesel can be produced by a standard transesterification procedure in which methanolysis of M. oleifera oil was conducted by a standard procedure employing a 6:1 molar ratio of methanol to vegetable oil for 1 hour at 60°C with 1 wt% NaOCH3 as catalyst. It high content of oleic acid (>70%) results into high cetane number of approximately 67, one of the highest found for a biodiesel fuel. The heat of combustion of MOME (M. oleifera methyl ester) is 40,092 kJ/kg which is comparatively more than European standard of 35,000 kJ/kg. MOME displayed a cloud point 18°C and pour point of 17°C, these values are rather high and resemble those of palm oil which also contains even higher amounts of saturated fatty acids. The kinematic viscosity at 40°C of MOME was determined to be 4.83 mm2/s agrees well with the viscosity values of both ASTM D6751 and EN 14214 given in table 2. Table 12 shows the fuel properties of drum stick methyl ester.
|
|
3.10. Cleome Viscose L. (Wild Mustard)
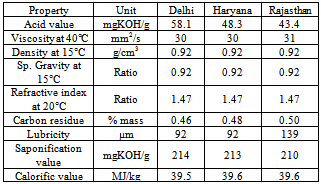
- After drying wild mustard in sun, it seeds yield varied between 22-26% of the fresh pod weight[19]. The seeds of Cleome viscose is available in large quantity from states like Rajasthan, Haryana and Delhi also areas of Aravali range. These plants grow in abundance in July after a monsoon rain and have life of about 13-15 weeks. The fatty acid profile obtained by gas chromatography contains palmitic acid, stearic acid, oleic acid, linoleic acid, saturated fatty acid and unsaturated fatty acids. Oil was extracted in hexane using the Soxhlet apparatus after mechanically crushing it. The use of H2SO4 as a catalyst facilitated both transesterification and esterification in the synthesis of biodiesel from the high acid value C. viscosa oil. The biodiesel produced from this oil fulfils the ASTM and Indian Standard requirements for vegetable oil-based biodiesels except in terms of oxidation stability. Thus this plant found to be potential short duration plant resource of non-edible seed oil for synthesis of biodiesel. Table 13 shows different properties of oil available from different states, which shows the diversity in fuel properties at different location of same country.
3.11. Brassica Juncea L. (Wild Mustard)
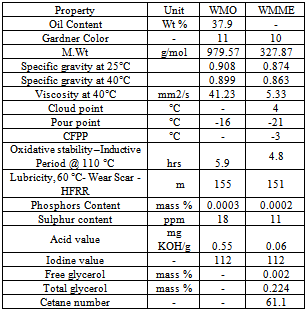
- Wild mustard (Brassica juncea L.) oil is evaluated as a feedstock for biodiesel production. Biodiesel was obtained in 94 wt.% yield by a standard transesterification procedure with methanol and sodium methoxide catalyst[20]. Wild mustard also known as field mustard, is an annual herbaceous plant belongs to Brassicaceae family that is an invasive agriculture paste (weed) to winter crops in southern and southern eastern Brazil. The oil content of wild mustard oil is about 38%. Wild mustard oil had a high content of erucic (13(Z)-docosenoic; 45.7 wt.%) acid, with linoleic (9(Z), 12(Z)-octadecadienoic; 14.2 wt.%) and linolenic (9(Z), 12(Z),15(Z)-octadecatrienoic; 13.0 wt.%) acids comprising most of the remaining fatty acid profile. The fuel properties like the cetane number, kinematic viscosity, and oxidative stability (Rancimat method) of the methyl esters was 61.1, 5.33 mm2 s-1 (40°C) and 4.8 h (110°C), respectively. The cloud, pour and cold filter plugging points were 4, -21 and -3°C, respectively. Other properties such as acid value, lubricity, free and total glycerol content, iodine value, Gardner colour, specific gravity, as well as sulphur and phosphorous contents were also determined and are discussed in light of biodiesel standards ASTM D6751 and EN 14214 as shown in the Table 14. Also reported are the properties and composition of wild mustard oil, along with identification of wild mustard collected in Brazil as Brassica juncea L. (2n = 36) as opposed to the currently accepted Sinapis arvensis L. (2n = 18) classification. In summary, wild mustard oil appears to be an acceptable non-edible feedstock for biodiesel production.
|
3.12. Balanites Roxburghii Planch (Dessert Date)
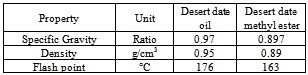
- As these seeds have low moisture content of 8.73% which is an indication of a reasonable shelf life for the seed, because there is little or no water for the hydrolysis of the oil to take place. The average oil content obtained is about 37.2%[21]. This is a dicotyledonous flowering plant that is popularly known as “Desert date” in English. It is widely grown in desert areas such as Africa, Middle East, India and Burma. The seeds of dates are going as waste and can be utilized in extracting oil and which can be used to produce biodiesel. Chemically, transesterification reaction involves the alkali group on the triglyceride (oil) is substituted with the methyl group of the alcohol. The base NaOH catalyst was dissolved in the alcohol to make it convenient for dispersing the solid catalyst into the oil. The percentage conversion of the oil to fatty acid methyl ester was 40.68%. The fuel quality parameters of the Desert date biodiesel such as the flash point and specific gravity are similar to those of Diesel. It is “readily biodegradable” compared with the diesel which is partially degradable. Table 15 shows fuel properties of desert date oil.
|
3.13. Aegle Marmelos (L.) Correa ex Roxb. (Bael Tree)
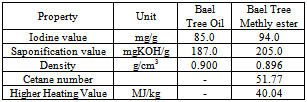
- The Aegle marmelos corre seeds yielded 49% oil. The molecular weight of the oil is 813.204 gram per mole based on the percentage of component fatty acids[22]. It is cultivated throughout India and also in SriLanka and northern Malaysia. Methyl esters of component fatty acids of Aegle marmelos corre seed oil contain Myristic acid, Palmitic acid, Oleic acid, Linoleic acid, Linolenic acid, and ricinoleic acid. Seeds were dried and oil is extracted using Soxhelet extractor with light petroleum ether for 24 hour. This seed oil is non-edible and is found to be the alternative feed stock for the production of bio-diesel since it convenes the major specifications of the bio-diesel. By-product of transesterification process leads to production of an important industrial product such as glycerol. If many of such plants are grown in large scale on waste land, then it can be an alternative to current conventional fuel. Table 16 shows the comparison of biodiesel properties of methyl esters of seed oil of Aegle marmelos corre with other biodiesel.
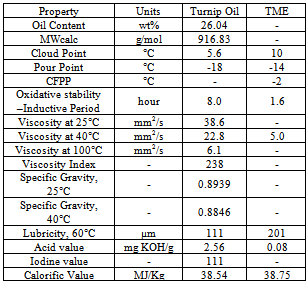
3.14. Raphanus Sativus L. (Turnip oil)
|
|
3.15. Gossypium Hirsutum L. (Cottonseed)
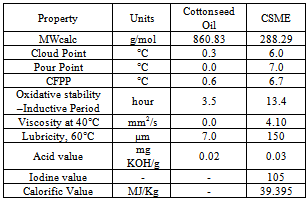
- Joshi et al.,[24] have currently studied the impact of the commercial additive on the Cottonseed oil methyl esters (CSME), where they have studied the low temperature operability and oxidative stability of cottonseed oil methyl esters (CSME) were improved with four anti-gel additives (Gunk, Heet, and Howe) as well as one antioxidant additive, (gossypol). Fuel properties of CSME and comparison to ASTM D6751 and EN 14214 biodiesel fuel standards are shown in Table 18.
|
3.16. Thlaspi Arvense L. (Pennycress)
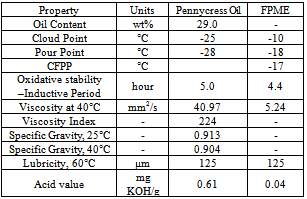
- Field pennycress (Thlaspi arvense L.) oil is evaluated for the first time as a feedstock for biodiesel production. Field pennycress (Thlaspi arvense L.), also known as stinkweed or French-weed, is a winter annual belonging to the Brassicaceae family. Native to Eurasia but with an extensive distribution throughout temperate North America, field pennycress is highly adapted to a wide variety of climatic conditions. The Brassicaceae family is a prolific source of biodiesel feedstocks, as evidenced by canola (or rapeseed, Brassica napus L.). Generally considered to be an agricultural pest (weed), field pennycress has potential to serve in a summer/winter rotational cycle with conventional commodity crops (such as corn or\ soybean), thus not displacing existing agricultural production. Field pennycress is tolerant of fallow lands, requires minimal agricultural inputs (fertilizer, pesticides, water), is not part of the food chain, is compatible with existing farm infrastructure, and has high oil content (20-36 wt %).[25] In addition, each plant may produce up to 15 000 seeds, and fields heavily infested with field pennycress are reported to yield up to 1345 kg of seed/ha. More recent results indicate that the yield from wild populations is in the range of 1120-2240 kg of seed/ha, which equates to around 600-1200 L of oil/ha versus 450 and 420-640 L/ha in the cases of Soybean and Camelina (C. sativa L.) oils, respectively.Bryan et al[26] has reported, preparation of field pennycress oil methyl esters (FPME) and evaluated their fuel properties, along with a comparison with biodiesel fuel standards such as ASTM D6751 and EN 14214. Biodiesel was obtained in 82 wt % yield by a standard transesterification procedure with methanol and sodium methoxide catalyst at 60 0C and an alcohol to oil molar ratio of 6:1. Acid-catalyzed pretreatment to reduce the acid value of crude field pennycress oil resulted in a yield after methanolysis of 94 wt %. Field pennycress oil had high contents of erucic (13(Z)-docosenoic; 32.8 wt %) and linoleic (9(Z),12(Z)-octadecadienoic; 22.4 wt %) acids with other unsaturated fatty acids comprising most of the remaining fatty acid profile. As a result, the methyl esters (biodiesel) obtained from this oil exhibited a high cetane number of 59.8 and excellent low temperature properties, as evidenced by cloud, pour, and cold filter plugging points of -10, -18, and -17°C, respectively. The kinematic viscosity and oxidative stability (Rancimat method) of field pennycress oil methyl esters were 5.24 mm2/s (40°C) and 4.4 h (110°C), respectively. Other fuel properties (Table 3.18) such as acid value, lubricity, free and total glycerol content, surface tension, as well as sulfur and phosphorus contents were also determined and are discussed in light of biodiesel standards such as ASTM D6751 and EN 14214. Also reported for the first time are cetane numbers of methyl esters of erucic and gondoic (methyl11(Z)-eicosenoate) acids, which were found to be 74.2 and 73.2, respectively. In summary, field pennycress oil appears to an acceptable feedstock for biodiesel production.
|
3.17. Mathematical Models for the Biodiesel
- In Recent past, Mitra et. al.[27] has reported Mathematical Modeling for the Prediction of Fuel Properties of Biodiesel from their FAME Composition. They have compared fuel properties like viscosity, density and High Heat Value (HHV) of ten biodiesels namely Corn, Cottonseed, Linseed, Rapeseed, Safflower, Soybean, Sunflower, Palm, Mahua, and Jatropha predicted using their FAME (Fatty Acid Methyl Ester) composition by regression analysis. The results obtained are compared and found to be in good agreement with reported literature values. Recently, Muazu et al has reported Development of Mathematical Model for Predication of Biodiesel yield using Jatropha oil as non-edible feedstock[28].
4. Application of Bio-diesel from Non-edible Seeds
- The fossil fuels are non-renewable and due to continue use, they will deplete in the future and there will be energy crisis in the world. Many experts believe that by the year 2070, the world will be exhausted of fossil fuels. The use of fossil fuels also pollutes the environment and causes environmental problems like global warming, green - house effect, air-pollution etc. Moreover, important fossil fuels like oil and gas are concentrate in some of the countries, resulting the remaining countries like India have to completely depends on such countries which are producing oil and gas. The price structure of these commodities is also decided by political equations besides usual trade laws. The import of oil and gas costs valuable foreign exchange which also affects the country economy. For example, our import bill for 2011-12 has shot up to $475 billion. Of this, nearly a third, or $150 billion, comprises crude oil imports. The trade deficit has blown up to $175 billion. Oil imports now comprise a gargantuan 85% of the country's total trade deficit.[29] Oil from vegetable seeds can be used as fuel without any modification in Compression Ignition engine. The comparative studies showed that non-edible oil is good alternative fuel in India. Without changing the engine technology blending of biodiesel is an interim approach to overcome the problems of short supply and emission. Experimental findings proved that non-edible biodiesel when blended with diesel from 10-20% shows similar engine performance as engine fuelled with conventional diesel[30]. Experimentation was conducted on 4-stroke, 4-cylinder indirect injection water cooled CI engine to understand the performance of engine. The fuel used was B20 (blend of 20% neem biodiesel and 80% petroleum diesel by volume). The load was varied from no load condition to maximum of 12 kW at constant speed of 1500 RPM. It was found that brake thermal efficiency was higher for biodiesel blend as compared to diesel. Also emission of CO, NOx, and SO2 is less with B20 fuel compared to diesel. The flash point of B20 was higher which enhance is safety during storage and transportation[31]. Performance study of an engine using Karanj Oil (K100) and blending karanj oil with diesel name K10, K15, K20 as a fuel was carried out. Specific fuel consumption increases with the increase in blend but K15 has minimum brake specific fuel consumption due to similar properties of fuel as diesel[32]. Performance and emission study on a single cylinder diesel engine using preheated mahua oil was carried out. Due to preheating to 130°C of mahuva oil viscosity of the oil is decreasing which not only enhanced the heat release rate but also improved the engine performance and emissions. NOx emission was marginally increased but preheated mahua oil can be used as diesel substitute under emergency as well as running situations[33]. The effect of neem oil and its methyl ester on a direct injected four stroke, single cylinder diesel engine show that at full load, peak cylinder pressure is higher, peak heat release rate during premixed combustion phase is lower, ignition delay is lower for neat neem oil and neem oil methyl ester when compared with diesel at full load. The combustion duration is higher, the brake thermal efficiency is slightly lower, reduction in emission in NOx for neem oil and its methyl ester along with an increase in CO, HC and smoke emissions[34]. Compression Ignition engine basically use diesel as fuel and are mainly used in industrial, transport and agricultural applications due to its reliability, durability and high fuel efficiency. Despite of its extensive use in industry high smoke and NOx emissions are major issues related to it. Unburnt hydrocarbon due to less availability of oxygen in the combustion chamber leads to emissions. An extensive study is done to increase the oxygen contain in the fuel by the means of adding additives to diesel. Biodiesel itself is having large oxygen content as compare to diesel which makes it less emissive as compare to diesel. Here are some work which shows the use of additives in diesel no such study is still observed in biodiesel. Oxygenated additive is a chemical compound containing oxygen. It enhance combustion process and reduce emission by addition in volume it also reduces the amount of crude oil consumption. Dimethyl carbonate (C3H6O3) often abbreviated DMC is having oxygen content upto 53.3 wt% having lower heating value of 15.78 MJ/kg and boiling point of 90°C which are much lower than that of diesel fuel. DMC and EGM addition to diesel changes the physicochemical properties of blends. Adding this in appropriate proportion it will improve the engine performance and emission characteristics[35]. Performance and emission test was carried out on 4-stroke multi-cylinder CI engine using DMC-EGM-diesel blends. 5% blend of DMC by volume gives higher brake thermal efficiency than that of diesel. Minimum CO and NOx emission is found for 10% blend of DMC in diesel. The blends of diesel with 15% DMC and EGM by volume is the best fraction for reduction of smoke[36].
5. Conclusions
- The potential for using oil of non-edible seeds as an alternative fuel for compression ignition engine has vided scope. Different kind of biodiesel is produced from non-edible seeds such as jatropha, neem, karanj, kusum, jojoba etc. There are around 78 non-edible species identified for the production of biodiesel but testing on engine is done with only few of them. As different oil had different chemical properties enough scope is there to carry out future work using any one of the non-edible seeds identified in section 2 of this paper according to Indian perspective. One can develop transesterification process for the given seed and produce biodiesel from it. Conducting performance analysis using that biodiesel caters the final product analysis which will be used in automobile engines. Very less economical study is found in the literature. If someone identifies a non-edible seeds, then study its cropping pattern which will help him to understand the growth yield of that seeds per hectare of land. From the available growth yield what will be the oil extraction from given quantity of seeds and what will be the cost encountered in transesterification process along with the cost analysis of by-product. This complete understanding of production of biodiesel from non-edible seeds will help in commercializing the product and will also help our economy by reducing the import of crude oil. Mathematical model for transesterification as well as different fuel properties of biodiesels are also discussed.
ACKNOWLEDGEMENTS
- The authors acknowledge their families for giving support for caring out research activity. Grateful thanks are due to the Dean, Faculty of Technology and Engineering and Head of Department of Mechanical Engineering and Applied Chemistry of Faculty of Technology and Engineering, The M S University of Baroda for allowing the use of their institutional facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML