-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2013; 3(1): 7-11
doi:10.5923/j.ep.20130301.02
Synthesis of Jatropha Oil based Biodiesel Using Environmentally Friendly Catalyst and Their Blending Studies with Diesel
Shailesh N. Shah1, Amit Joshi1, Anjali Patel2, Varsha P. Brahmkhatri2
1Applied Chemistry Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda Vadodara, 390 001, Gujarat, India
2Chemistry Department, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara, 390 002, Gujarat, India
Correspondence to: Shailesh N. Shah, Applied Chemistry Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda Vadodara, 390 001, Gujarat, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
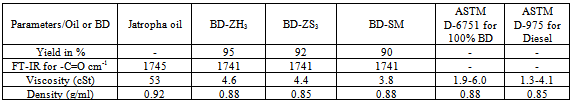
Jatropha oil is readily available as one of the cheapest non-food feedstock in India. In this work, we would like to explore the use of Zirconium based heterogeneous catalysts to synthesis Jatropha oil biodiesel ((JBD) and to take advantages of the solid nature as well as reusability of the environmentally friendly heterogeneous catalyst. In order to compare JBD was also synthesized using conventional sodium metoxide (NaOMe homogenous catalyst). Best yield was obtained using heterogeneous catalyst (ZH3). Synthesized JBD by both homogenous and heterogeneous catalysts were characterized by FTIR, and Diesel Fuel Properties like viscosity and density. Viscosities of synthesized all JBD were in agreement with ASTM D-6751 (100%-BD) and ASTM D-975 (100%-Diesel). In this study, Blending studies of Jatropha biodiesel-JBD (5 %) with diesel was also carried out and diesel fuel properties like viscosity, density, pour point and flash point were also investigated. Except density of blended mixture, all other fuel properties studied are in agreement with ASTM D-975 (100%-Diesel). 5% JBD Blend have nearly same physical properties as that of petro-diesel which demonstrates that it would be commercially viable to use in the field. This study will help biodiesel producer to be competitive in production of Jatropha based biodiesel, using heterogeneous catalyst and work up process would be easied and reusecatalyst. Thus it provides the economic pathway for the synthesis of eco-friendly biodiesel.
Keywords: Bio-diesel, Jatropha Oil, Non-Edible Feedstock, Heterogeneous, Homogenous Catalysts
Cite this paper: Shailesh N. Shah, Amit Joshi, Anjali Patel, Varsha P. Brahmkhatri, Synthesis of Jatropha Oil based Biodiesel Using Environmentally Friendly Catalyst and Their Blending Studies with Diesel, Energy and Power, Vol. 3 No. 1, 2013, pp. 7-11. doi: 10.5923/j.ep.20130301.02.
Article Outline
1. Introduction
- Due to increase usage of fossil fuels, we are in a position to go through scarcity. Goal of this study is to reduce the utilization of fossil fuels and obtain diesel from non-edible feed stocks without destructing supply from edible oil.This requirement has opened up the new door for energy sources from the bio-based materials like plant oil. More-over, due to famous food v/s fuel debate, scientists are considering non-food as bio-energy source to prepare bio-fuel like biodiesel. Biodiesel is Fatty Acid Methyl Ester obtained from plant oil after reacting it with Methanol in presence of homogenous catalyst like sodium methoxide1. However, homogenous catalyst has following limitation, which makes them environmentally non-friendly. a) Soluble in the reaction mixture which forms emulsion and to break , it requires long time b) Catalyst is not separated after reaction and c) Pure product with less yield. Heterogonous catalyst offers following advantages and make them more environmentally friendly a) Insoluble in the reaction mixture which offers easier work up process b) Catalyst can be separated easily-so one can reuse catalyst and c) More yield with less tedious purification process. Recently, we have reported preparation of biodiesel using transesterification of non edible feedstock like [Jojoba oil2, and Wild Brazilian mustard (Brassica juncea L.)3} with methanol using sodium methoxide as homogenous catalyst. In further study, we reported Jojobyl methyl acetate as other class of biodiesel, which was produced using direct acetylation of the jojoba alcohol4. We have also reported composition and physical properties studies of non-edible oils of field pennycress (Thlaspi arvense L.)and (Lepidium sativum L.) 5, arugula, shepherd’ spurse, and upland cress oils6. Jatropha oil is readily available as one of the cheapest non-food feedstock in India. With this in view, to explore the use of heterogeneous catalyst as Eco-friendly for synthesis of the Jatropha oil based bio-diesel (JBD), current work was undertaken. In this work blending studies of JBD (5%) with commercially available diesel was also proposed in order to demonstrate it’s viability for transport vehicle (real world application).
2. Experimental
2.1. Materials
- Jatropha Oil (CSMCRI = 870 g/mole), Dichloromethane (GR-MERCK) as a solvent, Methanol (GR-MERCK), Sodium Hyrdoxide pellets (MERCK). Petro-diesel was purchased from the petro outlet in Baroda, Gujarat, India. All the materials were used directly without any prior purification.The solid acid catalysts for Biodiesel synthesis, 12-Tungstophosphoric acid supported on hydrous Zirconia (ZH3) and 12-Tungstosilisic acid supported on hydrous Zirconia (ZS3) were prepared by the method reported in literature7-8. Catalyst-1 as 12-Tungstosilisic acid hydrous Zirconia (ZS3) and Catalyst-2 as 12-Tungstophosphoric acid hydrous Zirconia (ZH3).
2.2. Procedure
2.2.1. Preparation of Jatropha oil bio-diesel (JBD) using Heterogeneous catalysts (ZS3)
- The transesterification of Jatropha oil with methanol was carried out in a three necked round bottom flask of cap. 250 ml equipped with water condenser, mechanical stirrer, and a thermometer pocket. Jatropha oil sample (1 mole), methanol (6 mole), and 1.0 g of ZS3 with dichloromethane as a solvent was taken . The temperature of the reaction is increased to 60°C with continuous stirring at 1200 rpm for 4 h in oil bath. After, reaction was completed, reaction mixture was transferred into separating funnel. Glycerol was allowed to settle down by gravity. Glycerol layer was removed and dichloromethane solvent added in reaction mixture and was shaken . Organic layer was washed with distilled water till neutral pH=7 was achieved. With continuous stirring the organic layer was than poured over Sodium sulphate (Na2SO4) in flask and allowed to stand for 15 minutes.Than it was filtered . The excess of dichloromethane wass than evaporated using rotatory evaporator.The weight of the product was 7.6 g (using the ZS3 Catalyst) % Yield = 92 %
2.2.2. Preparation of Jatropha oil bio-diesel (JBD) using Heterogeneous catalysts (ZH3)
- The transesterification of Jatropha oil with methanol was carried out in a three necked round bottom flask of cap. 250 ml equipped with water condenser, mechanical stirrer, and a thermometer pocket.. Jatropha oil sample (1 mole), methanol (6 mole), and 1.0 g of ZH3 with dichloromethane as a solvent was taken. The temperature of the reaction was increased to 60℃ with continuous stirring at 1200 rpm for 4 h in oil bath.After, reaction was completed, reaction mixture was transferred into separating funnel. Glycerol was allowed to settle down by gravity. Glycerol layer was removed and dichloromethane solvent added in reaction mixture and was shaken . Organic layer was washed with distilled water till neutral pH=7 was achieved. With continuous stirring the organic layer was than poured over Sodium sulphate (Na2SO4) in flask and allowed to stand for 15 minutes.Than it was filtered . The excess of dichloromethane wass than evaporated using rotatory evaporator.The weight of the product was 7.8 g (using the ZH3 Catalyst) % Yield = 95 %.
2.2.3. Preparation of Jatropha oil bio-diesel (JBD-SM) using Homogeneous catalysts (30 % Sodium Methoxide solution in Methanol)
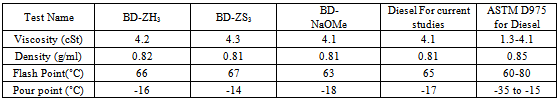
- 250 ml single necked round bottom flask equipped with the water condenser was taken on oil bath. 150 g of Jatropha oil, 25 gm of methanol , and10 g of Sodium methoxide solution in methanol was taken in above flask. The whole reaction mixture was allowed to stir for 4 hrs at 600 rpm on magnetic stirrer. The reaction temperature was maintained at 65 ℃. The reaction mixture was turned to creamish white in color. The whole mixture was washed with the brine solution to break the resultant emulsion formed during the reaction.The reaction mixture was further transferred to the separating funnel for separation of two phases. The upper phase consisted of methyl ester with small amount of impurities such as residual alcohol, glycerol and partial glycerides, while the lower portion consisted of glycerol. The upper methyl ester layer collected was further purified by distilling residual methanol at 60℃.The weight of the productwas 134.60 g (using the CH3ONa Catalyst) % Yield = 89.7 %The productwas further used for the functional group characterization using the Fourier Infrared Spectroscopy- FTIR (Figure 1 and 2) as well as Diesel Fuel Properties like kinematic viscosity, density, pour point and flash point as shown in Table 1.
2.2.4. Blending studies of Bio-diesel with Petro-diesel
- In Blending studies of bio-diesel with petro-diesel, 5.0 ml of the Jatropha based biodiesel (JBD) + 95.0 ml of petro-diesel was mixed and stirred vigorously for 30 minutes and tested for different fuel properties like viscosity, density, pour point as well as flash point and data were reported in Table 2.
2.5. Characterization
2.5.1. Fourier Infrared Spectroscopy- FTIR
- FT-IR spectra of the compounds were recorded on Shimadzu-8400S spectrophotometer by KBr pellet method. The spectra were obtained over the frequency range 4000-400 cm-1 at a resolution of 4 cm-1. The FT-IR spectra of samples are shown in Figure 1-2.
2.5.2. Diesel, Biodiesel and Blend Fuel Properties Studies by ASTM Method
- Viscosity in cSt, and Density (g/ml) of Diesel, Jatropha Oil (JO), Jatropha based bio-diesel (JBD) and it’s blend with Diesel fuel properties were characterized using ASTM D-6751 (Standard for 100% BD)1 and ASTM D-975 (Standard for 100% Diesel)10.
3. Results and Discussion
- Jatropha biodiesel (JBD) was synthesized using transesterification of Jatropha oil with methanol in presence of either homogenous catalyst (Sodium Methoxide, JBD-SM) or heterogeneous catalysts (ZS3, JBD-ZS3 and ZH3, JBD- ZH3 ). Characterization of the JBD for functional group determination was carried out using the using FTIR as shown in Figure 1-2.The IR spectrum (Figure 1) for Jatropha Oil (JO) shows the ester linkage of triglyceride at 1745 cm-1. The IR spectrum (Figure 2) of Jatropha Bio-diesel (JBD-ZH3) shows the ester linkage of Fatty Acid Methyl Ester at 1741 cm-1. When we compare the FTIR of the Figure 2 with the baseline - JO (Figure 1), it clearly shows that Carbonyl group of the JO is shifted from 1745 to 1741 cm-1 , which indicates that number of carbonyl group is decreased. JBD is Fatty Acid Methyl Ester containing only one carbonyl group compare to baseline JO, which originally contains three carbonyl groups This observation is in consistent with recently reported FTIR spectra of the JBD report by EPA9, which also supports that synthesized product is the bio diesel. tThere is no alcohol or un-reacted methanol which have broad absorption in the range of 3600-3500 cm-1. Same discussion is true for the other catalyst ZS3.
3.1. 100% Jatropha Based Bio-diesel
- Table-1 describes different parameters likes percentage yield of JBD, Carbonyl group frequency in cm-1 in FTIR, Viscosity in cSt, and Density (g/ml) of Jatropha Oil (JO) and JBD. It also shows comparison of Viscosity in cSt, and Density (g/ml) of Jatropha Oil (JO) as well as JBD with ASTM D-6751 (Standard for 100% BD)1 and ASTM D-975 (Standard for 100% Diesel)10.
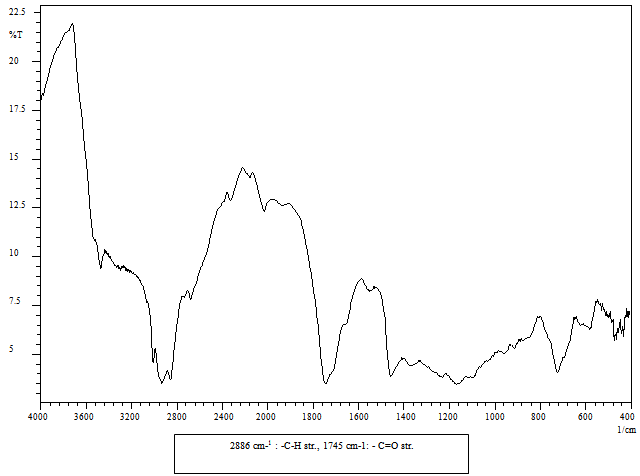 | Figure 1. IR Spectra of Jatropha Oil |
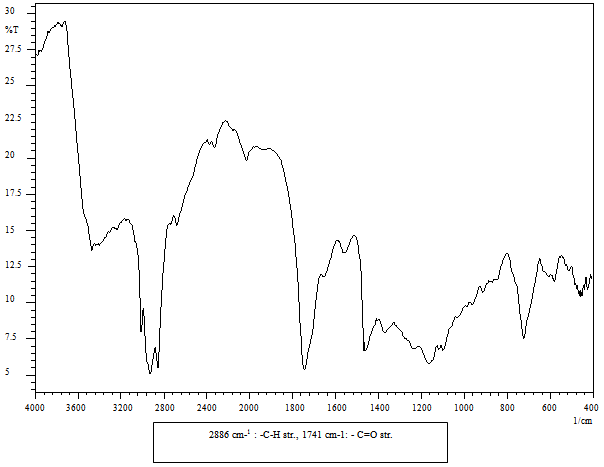 | Figure 2. IR Spectra of Biodiesel using ZH3 |
|
|
3.2. Blending studies of Jatropha biodiesel-JBD (5 %) with diesel
- Table-2 describes Blending studies of Jatropha biodiesel-JBD (5 %) with diesel and their different fuel properties like Viscosity in cSt, and Density (g/ml), Flash point (℃) and Pour Point (℃). It also shows comparison of Blending studies of Jatropha biodiesel-JBD (5 %) with diesel with ASTM D-6751 ASTM D-97510 (Standard for 100% Diesel).Except density of above mentioned blended mixture, all other properties studied are in agreement with ASTM D-975 (100%-Diesel). Thus 5% JBD Blend have nearly same physical properties as that of petro-diesel used for current studies which demonstrates that it would be commercially viable for use in the field.
4. Conclusions
- Current study has demonstrated the use of the heterogeneous catalyst based on Zirconium for the synthesis of the Jatropha oil biodiesel. 5% JBD Blend have nearly same physical properties as that of petro-diesel which demonstrates that it would be commercially viable for use in the field. This study will help biodiesel producer to be competitive in the production of Jatropha based biodiesel, using heterogeneous catalyst which has easier work up process as well as reusability of the catalyst. Thus it provides the economic pathway for synthesis of eco-friendly biodiesel. Further studies will continue using more heterogeneous catalysts and by blending higher percentage of JBD with petro-diesel and study their fuel properties .
ACKNOWLEDGEMENTS
- We would like to thank Head, Department of Applied Chemistry, Faculty of Technology, and, Head, Department of Chemistry, Faculty of Science, The M. S. University of Baroda for providing necessary laboratory facilities. We would also like to thank Mr. Vinod I. Bhoi, Department of Applied Chemistry, Faculty of Technology, M.S. University of Baroda, for recording FT-IR spectra of the samples. We would also like to thank CSMCRI, Bhavnagar for providing Jatropha oil and Supra Combines, Baroda for providing Sodium Methoxide catalyst.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML