-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2023; 13(1): 9-28
doi:10.5923/j.env.20231301.02
Received: Sep. 18, 2023; Accepted: Oct. 7, 2023; Published: Oct. 13, 2023

The Influence of Soil-Water Relations in Mangrove Forests on Ecosystem Balance
Sabrina Dookie, Sirpaul Jaikishun, Abdullah Adil Ansari
Department of Biology, Faculty of Natural Sciences, University of Guyana, Georgetown, Guyana, SA
Correspondence to: Sabrina Dookie, Department of Biology, Faculty of Natural Sciences, University of Guyana, Georgetown, Guyana, SA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Mangroves represent highly important coastal ecological systems on a global scale. Despite being highly examined ecosystems, the literature reveals several knowledge gaps regarding the impact of soil-water relationships in mangrove forests. This comprehensive literature review integrates extant studies on the impact of soil-water relationships within mangrove ecosystems at global and local levels, with the aim of identifying the factors that facilitate or impede their capacity to flourish productivity. Our findings demonstrate that various biogeochemical processes that take place in soil and water have an impact on the functioning and balance of mangrove ecosystems. These processes prompt mangroves to develop ecophysiological adaptations that enable them to mitigate the effects of harsh environmental stressors and changes, predominantly caused by anthropogenic activities. Alterations in both the physical and chemical properties of soil and water within mangrove ecosystems can have a direct impact on their distribution, density, and diversity. The review underscores the necessity of establishing appropriate policies and governance mechanisms for the protection and conservation of mangroves. The interplay between soil and water has a significant bearing on the functioning of mangrove ecosystems, with potential implications for productivity and functionality as anthropogenic and natural phenomena constantly alter their physicochemical properties.
Keywords: Dynamics, Ecosystems, Mangroves, Nutrients, Review, Soil, Water
Cite this paper: Sabrina Dookie, Sirpaul Jaikishun, Abdullah Adil Ansari, The Influence of Soil-Water Relations in Mangrove Forests on Ecosystem Balance, World Environment, Vol. 13 No. 1, 2023, pp. 9-28. doi: 10.5923/j.env.20231301.02.
Article Outline
1. Introduction
- The mangrove biomes (‘mangrove forests’ or ‘mangal’) which thrive in the intertidal zones of subtropical and tropical shorelines are distinctive and constantly evolving ecosystems (Spalding & Parrett 2019). Being described as a blue carbon ecosystem, they are also known to protect and stabilise coastal regions from the harmful effects of natural disasters, offer a variety of ecological goods and services and underpin many agricultural and economic activities (Kulkarni et al. 2018). Although they are among the most productive and ecologically diverse habitats on Earth, benefiting our environment, communities, and economies, mangrove forests suffer tremendous dangers, much greater than those of other forests, which can eventually transform their dynamic characteristics (Malik et al. 2017). The degradation of mangrove ecosystems is primarily attributed to human-caused disturbances, variations in the climate, and the decreasing availability of various natural resources (Mousavi et al. 2022). Modifications to the distribution, productivity and health of mangrove ecosystems can have a significant impact on the existence and richness of other organisms, as well as the health and productivity of other forested areas (Ghayoumi et al. 2022). While many elements can significantly affect the dynamics of forested ecosystems, the extensive interplay among vegetation and their surrounding soil and water may heavily influence mangrove habitat stability and quality (Maiti & Chowdhury 2013). The variability of both water and soil compositions in and surrounding mangroves is recognised to be a primary driver of both positive and negative changes in mangrove vegetation production, functioning, and survivorship (Wimmler et al. 2021). Saline and anoxic ecosystems present a physiological challenge for vegetation due to the notably adverse water potentials resulting in a less advantageous process of water uptake (Reef & Lovelock 2014). Mangroves have evolved the ability to tolerate high salinity and anoxia extremes through the adaptation of plant structures such as salt-excreting leaves and breathing 'stilt' roots (Srikanth et al. 2015). Soil physical composition, specifically the clay, sand, and silt ratios that define hydraulic conductivity and permeability as well as soil salinity and water content in the mangal ecosystem, have a direct impact on species composition and development (Torres et al. 2018). In addition to being responsible for the distinct chemical and physical circumstances of mangroves, hydroperiod conditions also influence a wide range of other aspects of mangrove ecosystems, including soil abiotic stress conditions, organic material deposition, species diversity and composition, and primary productivity (Wilwatikta et al. 2020). The interdependence of soil and water is a crucial aspect of mangrove ecosystems, wherein they serve as intrinsic agents in the deterioration and exhaustion of mangrove forests when exposed to human-induced pollutants (Cochard 2017). Although there are many reviews on the various ecological aspects of mangroves, there is no exclusive review detailing the relationships between soil and water, and their influence on mangrove forests. Within this context, the aim of this review is to highlight the current state of knowledge and provide a comprehensive foundation and analysis of the influence of soil–water relationships on mangrove ecosystems.
2. Materials and Method
- Our review was conducted by accessing relevant research on mangroves through the use of Google Scholar. The research publications which included specific keywords such as 'mangroves', 'forest ecosystems', 'carbon', 'mangrove soil', 'disturbed', 'undisturbed', 'porewater', and 'soil nutrients’ were considered for this review. The retrieval of journal articles was facilitated by utilising many databases and worldwide online journals, including but not limited to Jstor, EB-SCO host, Hindawi, Intechopen, Springer, Elsevier, and ResearchGate. The present study involved the selection of over 175 pertinent scholarly works surrounding the contemporary condition of mangroves at both local and global scales, as well as the many elements that might potentially influence their ecological processes during the time frame spanning from 2000 to 2023. Following the acquisition of articles from a global perspective, the search was further narrowed down to the literature specifically focused on soil - water relationships in mangrove forests. This was done in order to gain a comprehensive understanding of the present conditions of mangroves while also taking into account environmental factors that influence the relationship between soil and water in mangrove forests, and ultimately affecting their ecosystem balance. Our findings are presented in two sections focusing specifically on mangrove ecosystem balance under the influence of porewater constituents namely pH, TDS, EC, and salinity, and physicochemical properties of mangrove soil inclusive of pH, EC, salinity, N, P, K, Ca, S, Mg, Fe, Zn, Mn, and Zn concentrations, following the conceptual framework summarised in Figure 1.
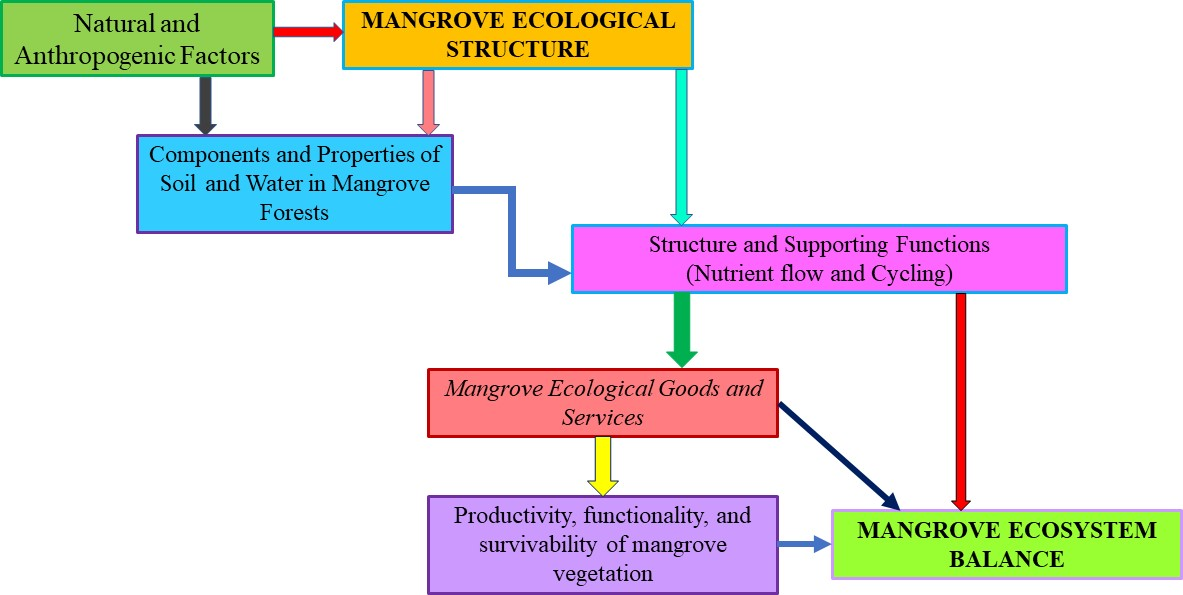 | Figure 1. Conceptual framework of review on soil–water relations on mangrove ecosystem balance |
3. Porewater in Mangroves Ecosystems
- The form and variety of water resources are recognised as one of the factors that influence the morphology of mangrove communities (Asakura et al. 2023). The hydroperiods of mangrove ecosystems serve as their hydrological distinctiveness, influencing the spatial distribution of nutrients and biogeochemical regulators of the soil (Pérez-Ceballos et al. 2020). The inflow of water into mangroves is influenced by the seasonal patterns of the water sources, whereas the outflow of water through mangroves is characterised by stronger currents compared to the inflow (Crase et al. 2013). Hydroperiod dynamics are essential for the structural and functional aspects of mangrove ecosystems as they are mainly accountable for the ecosystem's unique chemical and physical conditions, which affect a variety of factors, including soil anaerobic condition, organic material accumulation, species diversity and composition, and primary productivity (Torres et al. 2018). The lateral and vertical hydrodynamics are influenced by heavily forested mangrove trees, prop roots, leaves, and pneumatophores and may be unique due to their location (Megonigal & Neubauer 2019). There are two mechanisms through which tidal water in mangrove ecosystems can drain into waterways. The first mechanism involves the drainage of water utilizing swamp soil due to a difference in groundwater that exists between the creek and the swamp water while the second mechanism involves the flow of water via tidal flooding triggered by animal burrowing and tunnelling (Pérez-Ceballos et al. 2020). Additionally, studies have shown that alterations to the flow and composition of water can affect its quality and availability in mangrove ecosystems. Water contamination is mostly pointed towards polluted sediments, human habitation and activities, industrial effluent, agricultural and fertiliser runoff, oil spills, and municipal effluents (wastewater) (Gerolin et al. 2020; Donoso & Rios Touma et al. 2020). This can change the surface water quality, and the hydrological characteristics of numerous surface water sources, and channel platforms and has shown rapid increases in the emissions of greenhouse gases within the mangrove ecosystem which has raised significant concerns (Das et al. 2021). Furthermore, storms, sea level rise, and alteration of precipitation patterns often have a significant impact on mangrove hydrodynamic flow patterns (Pérez-Ceballos et al. 2020). The physicochemical characteristics of temperature, pH, salinity (electrical conductivity), and total dissolved solids (TDS), are also thought to be limiting factors for the survival of flora and fauna (Figure 2).
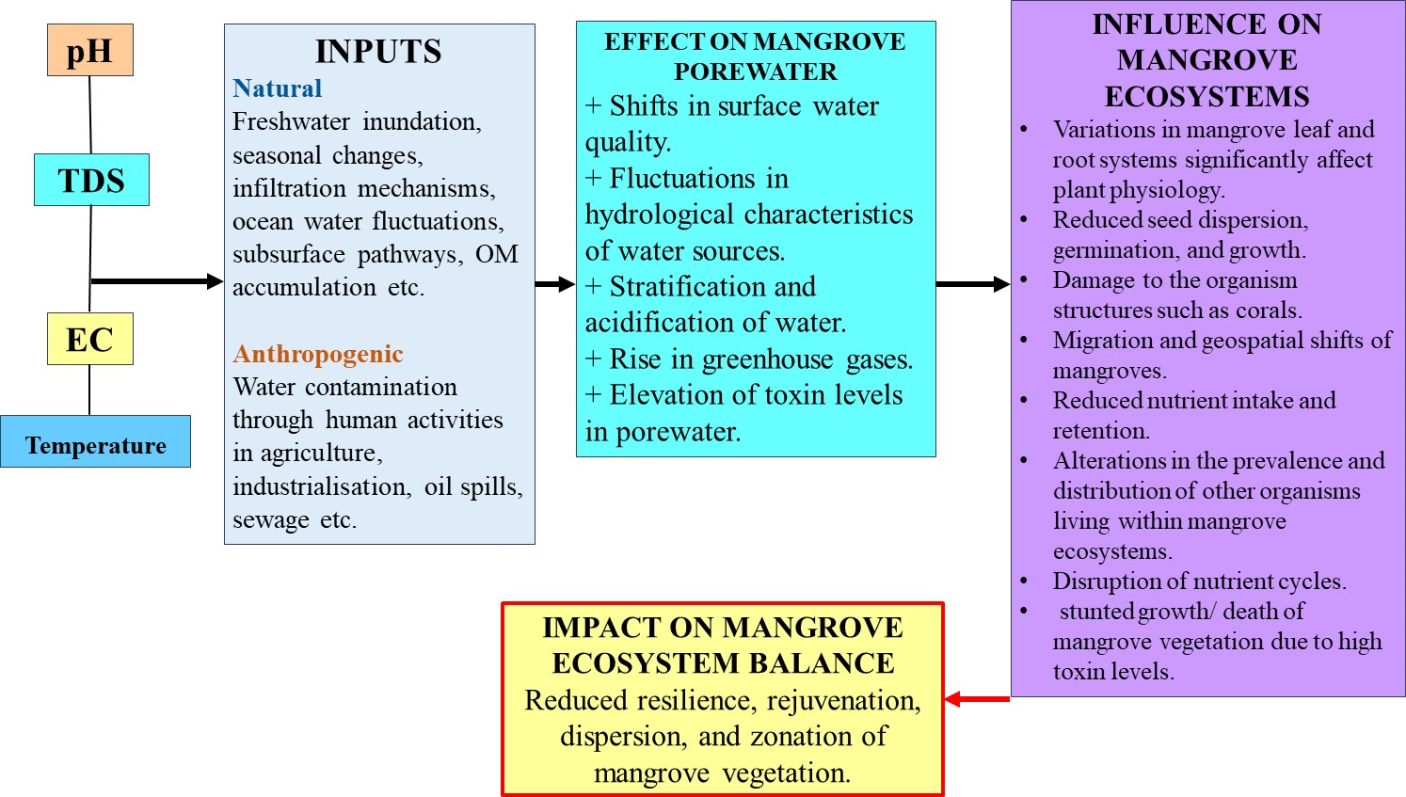 | Figure 2. Effect of water relations and components on mangrove ecosystem balance – synthesis of findings |
3.1. Temperature
- The temperature of the water can lead to ocean stratification which can have an impact on an organism's reproduction, metabolism, distribution, and development. Temperatures above 35°C have an impact on mangrove tree root systems, seed dispersal and growth, photosynthesis, and CO2 fixation (Jacotot et al. 2018), which may disrupt the patterns of vegetation dispersion, and to a greater extreme, contribute to heat-stressed phenomena ranging from changes in the phenology of organisms, to coral bleaching (Sucharit Basu et al. 2017). Additionally, the location, time of day, air circulation and flow, elevation, seasonality, and level of the water source all influence the temperature of surface waters (Lotfinasabasl et al. 2018). The phenomenon of climate change has resulted in significant variations in water temperatures, leading to the migration of mangroves to locations that are situated further inland or at greater elevations (Godoy & de Lacerda 2015). The variability in water temperature has an impact on the surface ocean circulation, which in turn affects tidal exchange and geospatial distribution of mangrove propagules, and constrains the functional ability of other ecosystems that are interconnected with the mangal community (Jennerjahn et al. 2017).
3.2. pH
- pH fluctuations can cause physiological strain to various species, resulting in reduced reproduction, growth, and overall health. Additionally, it can lead to a decline in biological diversity and stream community structure (Pawar et al. 2013). Hilmi et al. (2019) have reported on the optimal pH range for mangrove growth, which is found to be between 6.0 and 8.5. The rate of intake of nutrients and retention, as well as the accumulation of heavy metals like mercury and lead in mangroves, is impacted by fluctuations in water pH (Cabañas-Mendoza et al. 2020). This phenomenon is influenced by several factors, including bicarbonate decomposition, freshwater influx, and the presence of colloidal particles (Adedokun et al. 2013). On the other hand, it is worth noting that the water-based solution within the interstitial spaces of mangrove ecosystems can harbour a noteworthy quantity of trace elements. These elements may be discharged into surface waters as a result of fluctuations in the pH levels of the water (Holloway et al. 2016). The pH levels of water in mangrove stands are subject to variation based on factors such as depth, cyclical nature, and the stage of maturity of the forests (Figure 2). The ocean's pH has been observed to decrease as a result of increased combustion of carbon-based fuels and growing urbanisation, which has led to significant amounts of CO2 uptake by the ocean. This phenomenon has been linked to a rise in the frequency of ocean acidification (Liu et al. 2021). Mangroves are natural wetlands that serve as sources for sediment-based methane fluxes that are also regulated by water pH (He et al. 2019). Consequently, the pH levels of water may impact the prevalence and spatial dispersion of various aquatic fauna such as shrimps, fishes, crabs, and oysters. This relationship may be associated with the extent of environmental contamination (Redjeki et al. 2020).
3.3. Salinity
- The ability of mangroves to withstand salt water is associated with their superior efficiency in using water for primary growth compared to other types of woody plants. This enables them to achieve a remarkable level of productivity even in situations where freshwater is scarce (Lovelock et al. 2017a) (see Figure 2). Shifts in rainfall, flow through rivers, and evaporation demand, as well as competition for water among plants, are expected to have a notable effect on the rate of development and diversity of species in coastal areas, owing to variations in their salinity levels (Peters et al. 2020). The presence of high levels of salinity in the substrate of mangroves can lead to hydraulic disintegration and ion excess toxicity, resulting in reduced growth and survival. This highlights the importance of physiological responses to salinity as a potentially critical factor (Méndez-Alonzo et al. 2016). Furthermore, the dissolution of salts such as potassium and sodium chloride has a significant effect on the electrically conductive properties of water, which can be assigned to high concentrations of organic matter, dissolving salts, cations and anions, poor flow of freshwater, disposal of waste from households and businesses, and runoff from agriculture (Castro et al. 2018). The vertical distribution of soil water salt content in mangrove soil may exhibit seasonal shifts due to flooding and infiltration processes occurring on an everyday basis. This can facilitate the development of species of mangroves along a horizontal salinity shift extending from upstream to downstream (Komiyama et al. 2020). Environmental conditions influence salinity levels, which in turn affect the resilience, dispersion, and rejuvenation of mangrove vegetation. Depending on the species, mangrove plants may flourish in salt levels ranging from 0 to 35 ppt, and surpassing this limit can have a deleterious effect on mangrove forest vegetation (Bathmann et al. 2021). In general, mangroves are abundant whereas salinities are lower. At high salinity levels, mangroves use greater amounts of energy for sustaining the equilibrium of water and concentrations of ions than for development and primary production (Kodikara et al. 2017). The presence of saline water in the interstitial space has been found to have several effects on plant physiology, including a reduction in leaf area and photosynthesis, an increase in the pressure of osmotic fluid of leaf sap, an increase in the leaf area/weight proportion, and a reduction in the overall concentration of nitrogen, potassium, and phosphorus (Barik et al. 2017). In arid periods, the level of salinity on the surface tends to be greater than during rainy seasons, and it is twice as much as that of seawater. The availability of freshwater is of utmost importance for the growth of mangroves in supratidal zones, as it serves to mitigate the high salinity levels of groundwater (Prihantono et al. 2023). The salinity levels in mangrove ecosystems are influenced by their geographical location, as open and exposed areas tend to have greater rates of evaporation during dry periods compared to enclosed and sheltered areas (Dittman et al. 2022). The phenomenon of hypersalinity has a significant impact not only on the mangrove but also on the wider mangrove ecosystems. The decline and depletion of mangrove ecosystems have been found to have adverse effects on biodiversity, soil organic carbon and plant biomass (Zhu et al. 2021). Consequently, this phenomenon can cause mangrove forests to transition from being a sink for carbon to a source of carbon, leading to a rise in the release of greenhouse gases.
3.4. Total Dissolved Solids (TDS)
- The levels of TDS in the ecosystems of mangroves are subject to fluctuations caused by both upstream and downstream flows, as well as seasonal changes throughout the year. During the periods of monsoons, TDS values tend to be higher due to the presence of floating substances such as fine silt, which are carried by rainwater (Odigie & Olomukoro 2020). The forest structure can be affected by possibly challenging conditions, which are demonstrated by the seasonal fluctuations in TDS concentrations. TDS gradients in seawater also have a positive effect on sediment, as well as the levels of phosphorus and nitrogen in mangrove forests (Shaltout et al. 2020). Elevated TDS levels are linked to the existence of high organic compounds or human-induced actions, such as the infiltration of wastewater from domestic sewage or industry. The fluctuations in TDS in environments that are polluted have been observed to impact the abundance and response of communities of microorganisms (Inyang & Wang 2020). Elevated TDS levels caused by dissolved salts can have adverse effects on various forms of aquatic life. This is due to the dehydrating impact of the salts on the coverings of fish and other aquatic creatures (Akther et al. 2018). Variations in TDS within mangrove swamp areas can be observed across varying depths. On an ecological scale, the development and distribution of mangroves are significantly impacted by alterations in TDS concentrations. This is due to the resultant fluctuations in salinity levels and the heightened discharge of heavy metals into the surrounding environment which can have fatal consequences (Dey et al. 2022).
4. Soil in Mangrove Ecosystems
- Soil ecosystem activities are linked to soil biogeochemical cycles and reflect the extent to which mangroves are preserved or degraded, as knowledge of soil dynamics is useful for predicting ecosystem responses to changing environmental parameters (Andrade et al. 2018). In some instances, soil attributes are not directly responsible for the provision of ecosystem services, but they may act as intermediaries for the delivery of services surrounding the cycling of nutrients and water as well as soil biological activity (Vincente et al. 2019). Mangrove soil is composed of fine particles that are abundant in organic carbon and are often characterised by salinity, anoxia, acidity, and water saturation. Mangroves receive essential nutrients through sediment and water transport during tidal submersion, flooding on a seasonal basis, and storm events (Alongi 2021). The bulk of mangrove soils is made up of mud, which is a mixture of silt and clay, making it denser (Figure 3).
4.1. Physical Components of Soil
4.1.1. Soil pH, Cation Exchange Capacity (CEC), and Salinity
- The significance of soil pH is well acknowledged, since it exerts a substantial influence on several chemical reactions pertaining to vital plant nutrients, phytotoxic substances, and contaminants. The solubility of these elements, which in turn determines their biological accessibility and movement, is influenced by pH, either through direct or indirect means. Soils exhibit a certain degree of pH stability, however there are situations in which variations in water pH might induce alterations (Penn & Camberato, 2019). The chemical composition and pH of soil are influenced by the presence of negatively as well as positively charged ions in both the water and the soil. The regulation of various biochemical activities and the influence on nutrient availability are attributed to soil pH, making it a governing parameter in soils (Odutola Oshunsanya 2019). The pH levels of mangrove soils exhibit variability, with some soils being acidic or alkaline. However, the majority of mangrove soils are observed to be highly buffered, with pH levels in the range of 6 to 7. In certain locations, the pH levels of mangrove soils can be as low as 5 (Hossain & Nuruddin 2016). Furthermore, the pH level is impacted by the depth of the soil, whereby surface regions exhibit greater values in contrast to deeper regions. This phenomenon may be attributed to the presence of acidic brackish waters that result from the aeration of soil sulphates (Arianto et al. 2015). The pH levels in mangrove soils can function as indicators of pollution due to their susceptibility to the influence of both natural and human actions (Celis-Hernandez et al. 2022). In addition, the pH of the soil can be influenced by numerous factors such as the geological features of the surrounding area, climatic conditions, precipitation and temperature patterns, seasonal variations, levels of soil nitrogen, and the presence of soluble ions (Ferreira et al. 2022). The decomposition of SOC may be influenced by soil pH values, which can impact microbial respiration and activity. The soil pH is recognised as a crucial determinant of the accessibility of metals in the soil. The phenomenon of metal desorption in acidic soils is attributed to the heightened discharge of hydrogen ions, which leads to increased competition among metal cations. This, in turn, results in a rise in the level of concentration and solubility of metals in the soil solution, thereby facilitating their potential absorption by plant roots. (Cabañas-Mendoza et al. 2020).The soil's cation exchange capacity (CEC) plays a significant role in regulating the accessibility and supply of nutrients, adjusting soil acidity and alkalinity, and determining the eventual disposition of pesticides and toxic metals (Datta & Deb 2017). The CEC of soils exhibits variability across global mangrove forests, with values ranging from 10.63 to 34.75 meq 100 g–1. Higher CEC values are indicative of greater quantities of organic matter (OM) present in the soil, especially in diverse zones (Bomfim et al. 2018). The prevalent pH conditions and the depth of the soil are factors that can be associated with significant variations in CEC (Nurul et al. 2022). In comparison to old growth, re-established mangroves typically exhibit lower CEC owing to the presence of deeper and more fertile soil quality (Jeyanny et al. 2019). Certain regions exhibit elevated levels of CEC in mangrove ecosystems as compared to other forested regions, which can be attributed to the prevalence of Na+, Mg2+, Ca2+, and K+ ions. Soils possessing a high CEC, such as clay, exhibit superior nutrient absorption and provision capabilities compared to soils with a low CEC (Andrade et al. 2022).Similar to other plant species, the impact of salinity on mangrove physiological processes is widely acknowledged, and it is generally agreed that elevated salinity levels can impede the physiological functions of mangroves (Seedo et al. 2018). Certain species of mangroves display a beneficial relationship between salinity and density, while other species exhibit a significant decrease in densities, gross primary productivity (GPP), and diversity of species with an increase in salinity (Perera et al. 2013). The salinity of soil is subject to notable influence from various factors such as water flow patterns, tidal inundations, topographical characteristics, precipitation, and proximity to the shoreline (Van Tang et al. 2020). In addition, soil salinity exerts a substantial impact on the microbial community compositions in mangrove soils, thereby potentially affecting both soil productivity and arboreal development. The impact of salinity on nitrogen and soil organic carbon alterations in coastal wetlands is a significant factor, suggesting that the intrusion of salinity due to climate change may have a more extensive effect on the coastal biospheres (Wijeratne et al. 2022). The salinity of mangrove soil is subject to both temporal and spatial variation and is influenced by both coastal terrain and climate (Komiyama et al. 2019). Mangrove vegetation typically exhibits a luxuriant growth pattern under conditions of lower salinity. However, it is susceptible to damage when exposed to elevated levels of salinity. Certain species, such as Rhizophora mangle, demonstrate growth inhibition when exposed to high salinities, whereas others, such as Avicennia germinans, exhibit exceptional development under these circumstances (Devaney et al. 2021). Seasonal fluctuations in soil salinity levels within mangrove ecosystems may be impacted by the penetration of inundated water, leading to variations in the salinity of the soil and nutrient availability (Komiyama et al. 2019). In regions characterised by limited freshwater resources, the process of evapotranspiration can result in the accumulation of excessive salt levels in soil porewater within the intertidal zone. This can surpass the salinity threshold of mangrove trees, rendering them intolerant to such conditions (Lovelock et al. 2017) (Figure 3). The increased level of soil salinity in times of drought may diminish the capacity of roots to attain hydration, whereas an arid atmosphere could restrict the acquisition of water through foliar water absorption. The concomitant impacts can potentially lead to the withering of mangroves that thrive in hypersaline soils due to drought (Duke et al. 2017).
4.1.2. Soil Organic Matter (SOM) and Soil Organic Carbon (SOC)
- Soil organic matter (SOM) is any substance that has been a part of or created by living organisms and has been returned to the soil to decompose ranging from undamaged primary organic matter to the extensively degraded combination of materials called "humus". The majority of SOM comes from plant tissues while the remaining dry matter consists of C, O, H, and minute quantities of S, N, P, K, Ca, and Mg (Kästner & Miltner 2018). SOM makes up 1–5% of soil mass, yet it plays a significant role in soil health due to its substantial influence on soil characteristics, functionality, soil trafficability, and hydrological functions (Hatten & Lyles 2019). The primary contributors of SOM in estuarine ecosystems include detritus from terrestrial plants, soils transported by water movement, phytoplankton, water-based macrophytes, and microphytobenthos. The accumulation of SOM is facilitated in the semi-enclosed estuary and mangrove-forest environments, primarily due to the robust internal movement of SOM within these ecosystems (Ogawa et al. 2021). SOM is the world's greatest terrestrial source of organic carbon (OC), as well as a massive repository of all vital nutrients and a key contributor to aggregation stability and formation by lowering penetration, and discharge rates, and improving flood control (Jackson et al. 2017). The net outcome of inputs (litter formation) and outputs (decomposition) determines the quantity of OM in the soil. SOM facilitates a wider range of biological, chemical, and physical processes that are essential for maintaining crucial ecosystem functions. This includes its role in carbon sequestration, as well as its function as an important source of energy and nutrients for biotic organisms (Hoffland et al. 2020). The degradation of soil due to inadequate land management practises is primarily attributed to the significant reduction in SOM. Vegetation composition impacts SOM decomposition and is regulated by plant-mediated variables such as litter biochemistry, climate, and edaphic characteristics (Lewis et al. 2014). According to Balke and Friess (2015), mangroves in the tropics are classified into three broad groups: "minerogenic, low tidal range (35% SOM, 60 cm tidal amplitude); minerogenic high tidal range (35% SOM, > 60 cm tidal amplitude); and organogenic, low tidal range (> 35% SOM, 60 cm tidal amplitude)." As a result, mostly biodynamic mangrove sediments are found in coastal regions with low tidal range and suspended matter, while primarily mineral mangrove soils are found in areas with significant tidal range and suspended matter (Saavedra-Hortua et al. 2020). Furthermore, within mangrove-dominated soils, low C/N ratios indicated that the SOM was most likely not formed primarily from mangrove detritus but rather from marine-acquired OM (Luglia et al. 2013). SOM quantities in the low-density regions may be greater than in the high-density mangrove areas and may be improved with stand age since the greater productivity of trees can contribute to an increase in SOM due to increased detritus trapping and litter quantity (Hu et al. 2021). The transfer of OM between neighbouring habitats has a significant impact on the structure and development of coastal ecosystems (Kallenbach et al. 2016). This exchange also affects the role of the primary producers as suppliers of nutrients as well as energy in food webs. The pattern of distribution of OM in mangroves is significantly influenced by tidal transportation and riverine runoff (Signa et al. 2017). The interdependence of both nitrogen and carbon cycles in coastal ecosystems is facilitated by denitrification, a process that necessitates the availability of OM in the form of detritus. However, this process can also restrict the generation of organic matter by means of nitrogen removal (Eyre et al. 2013) (Figure 3).
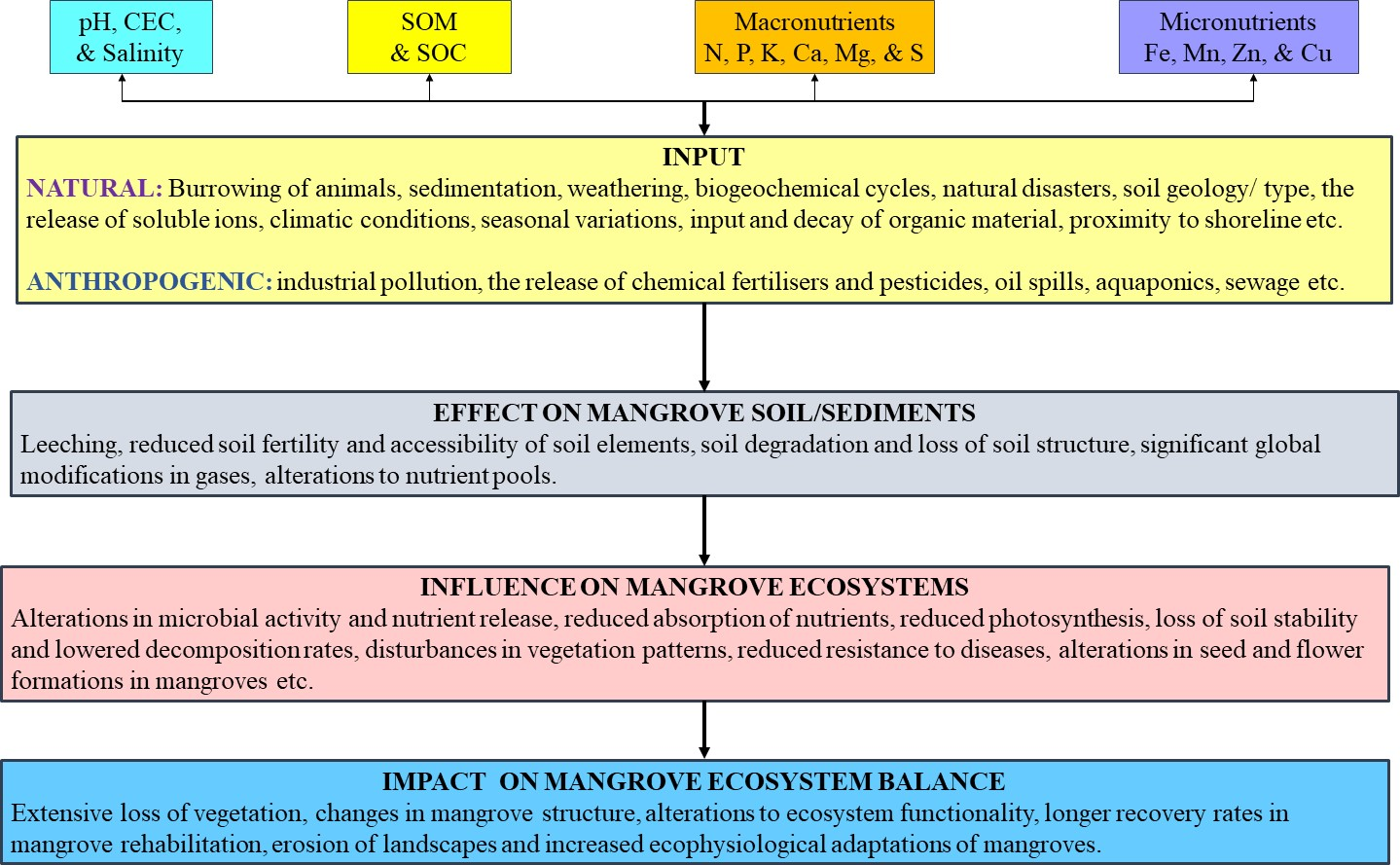 | Figure 3. Effect of soil relations and components on mangrove ecosystem balance – synthesis of findings |
4.2. Nutritional Components of Soil
4.2.1. Nitrogen (N), Phosphorus (P), and Potassium (K)
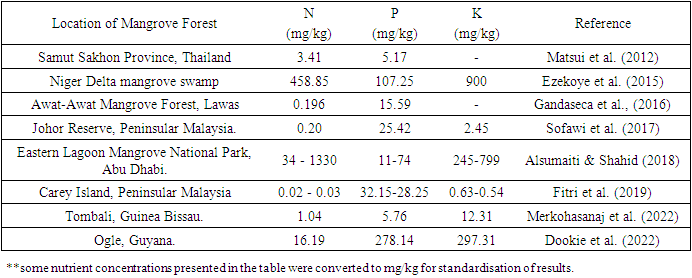
- Mangrove forests' anoxic soils, which are rich in organic material, are conducive to nitrogen (N) fixation and can serve as a significant source (Alongi 2021). The intricate bacterial processes occurring within the narrow oxic and anoxic zones of mangrove soil play a crucial role in determining the levels of N present in mangrove ecosystems. Elevated denitrification rates are attributed to the presence of denitrifying bacteria, resulting in the depletion of nitrite reservoirs and the generation of ammonium, which is the predominant variety of nitrogen detected in mangrove soil (Balk et al. 2015). The global average N stock in mangrove forests is 52.03 Mg N ha-1, with tropical terrestrial ecosystems contributing 96% of the total nitrogen (Alongi 2020). The efficiency and dynamics of mangroves are likely to be significantly impacted by global changes that influence the rates of ammonification, nitrification, fixation of nitrogen, and nitrate reduction, such as extensive soil disturbances (Alongi 2018). The process of burial serves as a noteworthy mechanism for the preservation of nitrogen, resulting in a considerable proportion of nitrogen input. The net primary productivity of mangrove forests is significantly influenced by factors that impact the below-ground development of roots and plant litter. This is due to their effect on tidal exchange and hydrodynamics, which in turn influence the equilibrium amount of nitrogen and inevitably the survival of these forests (Alongi 2018). The primary variables limiting N transformation processes in mangrove sediment are inorganic dissolved nitrogen's accessibility and microorganism immobilisation (Reis et al. 2016). The sediments of mangrove ecosystems predominantly function as a supplier of ammonium while concurrently acting as a repository for nitrates. Strong mineralization results in an increase in ammonium in pore water, whereas denitrification controls the elimination of nitrates and N2O production (Wang et al. 2021). In addition, the introduction of nitrogen by human activities can have a significant impact on the nitrogen cycle, leading to direct consequences for the functioning of ecosystems and potential indirect consequences for the structure of mangrove forests (Wang et al. 2019). (Figure 3). The N flux in mangrove ecosystems exhibits temporal and spatial variability, which is influenced by a range of factors such as litterfall, sediment, plant nitrogen requirements, environmental conditions, and river runoff (Wang et al. 2019) (Table 1). The duration of N immobilisation in intact mangrove locations is twice as long as in recovering locations. Furthermore, the total release of N after immobilisation is less rapid in intact sites, indicating a more conservative approach to N cycling. These findings suggest that intact mangrove ecosystems may be less disturbed and more stable (Marquez et al. 2016). Datta and Deb (2017) noted that managed mangroves exhibited a higher availability of soil nitrogen in certain regions compared to unmanaged mangroves. Mangroves in a pristine state have the ability to function as a nitrogen sink, whereas mangroves that have been impacted or eutrophied may serve as a nitrogen source. The efficiency of nitrogen removal exhibits variations based on latitude, wetland classification, and nitrogen loading. Nitrogen removal is higher in freshwater and naturally occurring wetlands as compared to tidal and artificially created wetlands (Rao et al. 2019). The impact of mangrove litterfall on nitrogen dynamics has been documented in the literature and is observed to vary between recovered and natural environments due to differing levels of interaction with human-caused disturbances (Mandal et al. 2013). In addition, the existence of fauna, including crabs, has the potential to impact the nitrogen dynamics within mangrove ecosystems, as they have the ability to stimulate higher levels of soil nitrification through direct means (Cheng et al. 2020). The salinity-induced increase in salt concentration in the soil has been observed to have a significant impact on the concentration of nitrogen in the roots and leaves, resulting in a sharp decrease. The inclusion of salt can induce modifications in the root anatomy, resulting in a notable decrease in absorption rates (Zhao et al. 2019). Mangroves serve as significant reservoirs that have the capacity to sequester substantial quantities of phosphorus (P), which undergoes conversion for utilisation in two distinct categories: (1) abiotic processes such as precipitation, dissolution, and chemisorption; and (2) biotic processes including hydrolysis, excretion, and assimilation (Alongi 2021). P is a crucial macronutrient that is essential for the biological development and growth of vegetation. The predominant forms of P present in soil are mineral phosphorus and organic phosphorus (Vass et al. 2015). The precipitation of P in mangrove sediment is typically attributed to its interaction with different cations present in the interstitial fluid. The naturally occurring P cycle is significantly influenced by microorganisms which facilitate the conversion of insoluble P to a form that is accessible to plants. This conversion is crucial for the continued development and survival of plants (Torres et al. 2019). P nutrition is linked to various factors such as seed and flower formation, stem strength, root development, crop maturation, and resistance to plant diseases. The rate of decomposition of organic material in sediment and the exchange of nutrients are also influenced by activities such as consumption, burrowing, and ventilation (Behera et al. 2017). In addition, certain marine organisms such as shrimps, lobsters, worms, bivalves, and bony fish play a significant role in the cycling of phosphorus. The bioturbation processes performed by these creatures are known to regulate the cycling of nutrients and P throughout the sediment (Tian et al. 2022). Anthropogenic pollution resulting from power plant wastewater, pesticides, heavy metals, and other industrial pollutants, eutrophication caused by fertilisers and sewage, and oil spills are significant issues that affect the distribution and cycling of phosphorus (Sarker et al. 2021). Furthermore, the concentration of P is subject to the influence of physio-chemical factors, including pH, accessible sulphur compounds, alkalinity, redox state, and the activities of microbiota and macrofauna (Sofawi 2017). The release of effluents containing P from human activities, particularly from sewage and aquaculture facilities such as fish and shrimp farms, poses significant eutrophication hazards to both marine and terrestrial mangrove ecosystems (Wei et al. 2022). The alteration of the P cycle is a well-known consequence of deforestation, modifications to hydrology during the building and operation of impoundments, shifts in land use, and the introduction of sediment and nutrients (Alongi et al. 2018). Potassium (K) is a crucial element for various plant functions, including the regulation of photosynthesis, processing of plant sugars, intracellular osmotic regulation, enzyme activation, and the production of proteins (Kumar & Kumara 2020). Additionally, it plays a vital role in plant defence against oxidative damage and in the preservation of osmotic equilibrium (Lu et al. 2016). Maintaining appropriate levels of K ions within plant cells is imperative for the appropriate response of plants to diverse forms of stress, including but not limited to salinity, drought, flooding, or herbivory (Zhu 2016). K is the second most prevalent nutrient found in leaf biomass, following nitrogen. This underscores its significant involvement and inescapable contribution to the development of plants. At the level of plant communities, the availability of K serves as a limiting factor for community growth (Alongi 2021). The accessibility of K in soil for root uptake can be classified into three distinct categories: assimilation into soil water, deposition onto clay and organic material particles, and retention within feldspar and mica crystal formations (Sardans & Peñuelas 2021). The utilisation of K-solubilizing bacteria is crucial in facilitating the availability of potassium for plant absorption (Mehak et al. 2022). Due to the slightly acidic nature of these soils, seasonal and site-specific variations in K concentrations can have an impact on the diversity of bacteria in mangrove substrates (Behera et al. 2013). Certain regions that harbour vital nutrients, such as K, exhibit a greater abundance of mangroves with elevated aboveground biomass (Constance et al. 2022) (Table 1). Furthermore, with regard to the restoration of mangrove ecosystems that have been damaged, discrepancies in nutrient levels, including K, may have an impact on the organisms that are linked to these areas and impede the future recovery of mangroves over an extended period (Krishnapriya et al. 2023). Generally, natural sites that have experienced limited disturbances exhibit higher K values in comparison to infertile and deteriorated sites. This observation may be attributed to factors such as higher levels of leaf litter, greater water input, and the type of soil, with clayey soils retaining more nutrients (Natarajan et al. 2022). Anthropogenic activities, including aquaculture, wastewater, and heightened utilisation of chemical fertilisers, have the potential to modify the potassium concentration in mangrove sediments (Krishnapriya et al. 2023), thereby influencing the ecological dynamics of these mangrove ecosystems.
|
4.2.2. Sulphur (S), Magnesium (Mg), & Calcium (Ca)
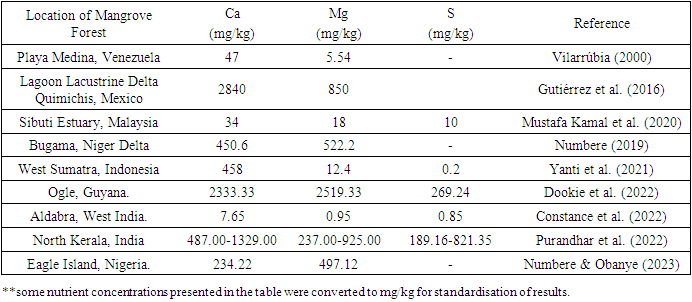
- Sulphur (S) is an essential constituent for the growth and maturation of plants, ranking fourth in importance among nutritional elements following NPK (Li et al. 2020). The assessment of soil S status is a crucial aspect that offers significant insights into its ability to be absorbed and the possible environmental implications. The dynamics of S in mangrove ecosystems are governed by the predominant redox environment and high organic matter levels, which are attributed to the presence of associated sulphur-reducing bacteria (Maurya et al. 2022). The forms of S can be influenced by various soil properties, including but not limited to N, P, Ca, Mg, K, Na, OM, pH, sand, silt, and clay (Uzoho et al. 2017). Magnesium (Mg) is involved in numerous biochemical and physiological processes, such as the process of photosynthesis enzyme activation, the creation of proteins, and the formation and manufacturing of chlorophyll (Chen et al. 2018). The influence of soil texture on the availability of Mg is well-established in the literature. Soils with a higher clay content tend to offer sufficient Mg for plant requirements, whereas soils with higher sand proportions do not (Senbayram et al. 2015). The release of plant-available Mg from soils is influenced by several significant variables, such as the duration and degree of the weathering process, soil moisture, soil pH, and root-microbial action throughout the soil (Gransee & Führs 2013). The transport of Mg within the soil is subject to a variety of factors, including precipitation, the composition of the soil, and the utilisation of synthetic fertilisers and lime amendments (Senbayram et al. 2015) (Table 2). Additionally, calcium (Ca) is necessary for growth and development, regardless of whether the plant is under stress or not. It serves as an additional messenger in various developmental and biological contexts, as well as in plant responses to surrounding stressors (Thor 2019). Ca has been demonstrated to be a crucial indicator of plant salinity tolerance. It plays a vital role in regulating ionic transmission and selection, stabilising cell wall structures, managing cell wall enzyme function, and controlling ion exchange capabilities (Wei et al. 2018). Ca is known to regulate several soil parameters, including pH and bicarbonate balance, and is closely associated with both inorganic and organic C, which accounts for its contribution to the diversity of microbes (Skariah et al. 2023). The presence of seawater serves as an inherent supplier of Ca for the soils in mangrove ecosystems. The quantity of CaCO3 present in deposits is subject to various factors such as its origin, the extent of dilution caused by the clastic influx, and the physicochemical forces that influence its characteristics (Nóbrega et al. 2014). Furthermore, there exists a potential correlation between nutrient proportions of S, Ca, and Mg in sediments and the levels of nutrients in various plant parts of mangrove species, including both trees and seedlings (Mustafa Kamal et al. 2020). Disturbed sites exhibit greater amounts of Ca and Mg compared to undisturbed sites, potentially attributed to robust tidal cycles (Gutiérrez 2016). The levels of Ca, Mg, and S in soil are typically higher during the monsoon period, which is attributed to the existence and functioning of roots as well as the tidal replenishment of soil (Alongi 2018). Contrary to the commonly held notion that the alkalinity of mangrove sediments may be attributed to the abundance of Ca that has been dissolved from shells and corals, research suggests that the pH level of soil can be somewhat acidic due to the activity of sulphur-reducing bacteria (Varon-Lopez et al. 2013). The quantity of exchangeable bases in soil, such as Ca and Mg, may exhibit a correlation with pH and CEC values. This correlation can be attributed to the origin of soil charges, which stem from the substantial amounts of OM and mineral colloidal material present in sediments (Madi et al. 2015). Furthermore, the dispersion of Mg2+ and Ca2+ ions is known to enhance cellulase production by microorganisms that inhabit mangrove sediments (Naresh et al. 2019). Elevated levels of Mg, Ca, and S are positively correlated with increased above-ground biomass in mangrove ecosystems situated in lagoonal environments. During particular times of the year, research has shown that interior mangroves possess higher levels of S, Ca, and Mg, while riverine mangroves have lower values, suggesting elevated environmental stresses in mangroves found inland (Constance et al. 2022) (Table 2). The composition and arrangement of vegetation from mangroves in secondary mangrove ecosystems, which are expected to remain mature forests, are primarily influenced by soil nutrients Mg and S. The amount of S and Mg in the continually growing biomass of trees was observed to increase with age in both the roots and leaves (Cooray et al. 2021). The presence of elevated levels of S, Ca, and Mg in the substrate of mud creatures such as lobsters and crabs, coupled with a low pH, suggests that they play a significant role in the acidification of sediments and the continued supply of exchangeable cations. These processes have the potential to induce the development of acid sulphate soil within the mangrove ecosystem (Hossain et al. 2019). The levels of Ca, Mg, and S exhibit fluctuations in response to natural occurrences, such as the combustion of vegetation in arid areas during instances of elevated temperatures (Numbere & Obanye 2023) (Figure 3).
|
4.2.3. Micronutrients/Trace Metals – Iron (Fe), Manganese (Mn), Copper (Cu), and Zinc (Zn)
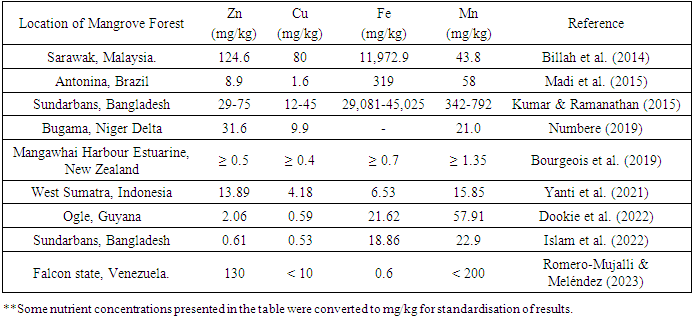
- Mangrove soils play a crucial role in mitigating pollution, particularly with respect to metal ions, through multiple mechanisms such as desorption, precipitation, absorption, dispersion, chemical transformations, and biological processes (Biswas et al. 2018). Manganese (Mn) is an essential element for the development and growth of plants, playing a significant role in chloroplast production, nitrogen metabolic processes, photosynthesis, and the manufacturing of riboflavin, ascorbic acid, carotene, and specific enzymes (Alongi 2021). Copper (Cu) is an essential element for vegetation, as it plays a crucial role in the production of seeds, chlorophyll synthesis, and various enzymatic purposes. On the other hand, Zinc (Zn) is also vital for plants as it contributes to the proper growth of plant resistance and functioning (Alongi 2021). Iron (Fe) plays a crucial role in various biological processes such as chlorophyll formation, nucleic acid metabolic processes, Fe-S protein, heme protein, and N2-fixation, and serves as the structural component of porphyrin molecules. Additionally, it is closely associated with the biogeochemical patterns of C, N, and S (Alongi 2021). The concentration of Mn in leaves from mangroves is the highest, followed by both living and deceased roots. On the other hand, Cu and Zn are predominantly present in the living and dead root systems of mangroves (Fones & Preston 2013). Plants employ micronutrients such as Mn, Zn, Cu, and Fe as the primary components of their defensive systems. Trace metals are crucial to aquatic life up to a particular concentration level. Nevertheless, their concentration level in the natural environment can become hazardous and toxic (Printz et al. 2016) (Figure 3). Various geochemical forms of metals, including Zn, Cu, Fe, and Mn, are dispersed in soil-water systems, affecting their ability to dissolve, accessibility, and potential for toxic effects. The levels of these metallic elements are significantly influenced by factors such as soil pH, the texture of the soil, organic carbon, and redox capacity (Islam et al. 2022). Furthermore, regardless of their capacity to endure and immobilise small amounts of metals, the depletion of mangrove soils could lead to the immobilisation of said elements, causing mangroves to transition from serving as a metal sink to a metal source (Costa-Böddeker et al. 2020). Various physiological and biogeochemical phenomena, including land use and land cover, watershed run-off, agriculture, fisheries, recreational activities, tidal behaviour trends, and microbial activity, influence the distribution of trace elements in intertidal soils. These processes exhibit important temporal and spatial variations and are interrelated with coastal hydro-meteorological sequences (Siddique et al. 2014) (Table 3). Zn and Cu, comparable to Fe, are present in trace quantities within the interstitial water layer and solidified form of natural mangrove soils. The intricate biogeochemistry of mangrove deposits and the presence of trace amounts suggest limited bioavailability and growth constraints (Alongi 2017). Notwithstanding the relatively high levels of heavy metals found in mangrove ecosystems, it has been observed that mangrove plants exhibit a preference for assimilating Cu and Zn while generally avoiding other types of heavy metals (Dudani et al. 2017). Fe concentrations hold significance in the acid sulphate soil locations, while Mn concentrations exhibit greater values in the non-affected sites due to the redox geochemistry and physicochemical characteristics of the soil that are associated with drying (Tognella et al. 2022). Soil types of mangroves that exhibit diminished Eh and current circulation in the upstream regions of waterways have a propensity to accumulate a greater quantity of macronutrients and trace metals, such as Zn, Fe, and Cu, in comparison to those located downstream. This phenomenon leads to the translocation of trace elements from the subsurface towards the surface of the soil, specifically in the form of litterfall (Bourgeois et al. 2019). Fluctuations in trace metal concentrations are indicative of alterations in the soil's geochemical surroundings and the amount of metal resulting from hydrological dynamics. These changes were found to contribute to the extensive loss of mangrove vegetation (Sohaib et al. 2023). Variations in heavy metal levels within disrupted mangrove ecological systems may be attributed to severe factors such as the uninterrupted release of heavy crude and industrial waste products, which contaminate groundwater as well as sediments, and organisms that inhabit mangroves (Numbere 2020) (Table 3). The rise in hydrodynamic energy conditions, caused by both natural and human-caused processes such as the building of dams, freshwater redirection, and irrigation processes, may also be responsible for the enrichment of Fe, Zn, Mn, and Cu (Conrad et al. 2023). The intensification of coastal degradation of mangroves due to increasing water levels and tides has resulted in reduced metal accumulation. The diminution of precipitation exacerbates the consequences of sea level escalation, fosters the erosion of seawater, and has the potential to amplify the aforementioned effects of metal concentration in sediments (Tang et al. 2022). Mangrove ecosystems in proximity to town areas are notably affected by untreated residential wastewater, farming activities, vessel discharges, and industrial run-offs which introduce traces and pollutants (Passos et al. 2021). The presence of mangrove vegetation and species in environmentally fragile regions may contribute to a reduction in metal pollution, as they are known to effectively regulate the accumulation of metals and maintain water quality. The reduced build-up of heavy metals in soil particles could potentially be attributed to the capacity of species of mangroves to efficiently absorb metals from such sediments (Sarath & Puthur 2021). The escalation of metal pollution, specifically Fe, Cu, Zn, and Mn, has been found to result in a range of biochemical and physiological modifications that impact the growth, metabolism, and cellular composition of plants (Nguyen et al. 2020b). Variation in the accumulation of metals among different species and tissues of organisms, such as crabs, was observed in both the extent of the accumulation of particulate metals and the particular tissues where they were predominantly present (Zhang et al. 2019). Mangrove trees possess the capacity to sequester metals, facilitating the translocation of these compounds from sediment and accumulating them within their tissues. As such, they can function as a mechanism for the confinement and extraction of contaminants (Nguyen et al. 2020). Fluctuations in concentration factors of Zn, Mn, Cu, and Zn in the tissues of mangrove plants may indicate the possibility of active absorption and storage of these metals for the purpose of promoting plant growth and development (Harcourt et al. 2015). Elevated levels of metals in the foliage of certain mangrove varieties have been linked to diminished levels of chlorophyll-a and chlorophyll-b, decreased carbon integration, and modifications in their physiological reactions (D'Addazio et al. 2023). The presence of tannins within the leaf litter of mangrove trees has the potential to form chelates with metals, thereby influencing their movement and alteration within the mangrove wetland ecosystem (Lang et al. 2022).
|
5. Research Gaps and Recommendations for Future Research
- Mangroves have been the subject of extensive studies across various themes and research domains. However, despite these efforts, there are still gaps in our knowledge about them. Our review highlights the need for further research to enhance the veracity of nutrient cycling and flow as well as the contamination of water in diverse mangrove ecosystem dynamics. This will facilitate a better comprehension of their effects on less explored areas and species. Further elucidation is required regarding the extant carbon sequestration potential of mangrove ecosystems as well as the determinants that may facilitate or hinder their efficacy in the face of diverse forms of perturbation, including but not limited to climate change. Alterations in the soil-water interactions within mangrove ecosystems have the potential to significantly impact their ecological dynamics, leading to disruptions in their overall structure as well as their functioning. Further investigation is required to ascertain the degree and nature of adaptability in the morphology and overall functionality of mangroves across diverse climatic zones and latitudes, as well as the impact of human interference on their behaviour. The ongoing escalation of climate change is exerting significant effects on mangrove communities and their corresponding geomorphological and ecological conditions at regional scales, with factors such as sea level rise, heightened storm activity, and rising temperatures being particularly influential. The relationship that develops between soil and water in mangrove ecosystems is influenced by various factors, including the resilience and dynamic modifications of these ecosystems in response to climate change. These factors operate temporally and spatially, magnifying their interactions and impact. Due to their dominance in tropical and subtropical regions, which are anticipated to undergo significant alterations in climatic patterns, mangroves are poised to encounter the most severe brunt of climate change effects, owing to their intertidal positions and susceptibility to environmental fluctuations. Our findings, therefore, suggest that to combat climate change and human advancement in the natural existence of these ecosystems, more research on the preservation, rehabilitation, and reforestation of mangroves are considered highly beneficial investments for the preservation of biodiversity.
6. Conclusions
- The interplay between soil and water relations can exert significant impacts on mangrove forests, both directly and indirectly. Our findings show that alterations to the physicochemical composition of porewater as well as mangrove sediments can impact the overall density, distribution, growth, morphology, restoration, and efficiency of mangrove vegetation. Soil–water relationships also affect the surrounding flora and fauna, especially soil organisms and microbes present within the mangrove forests themselves, which ultimately affects their biodiversity. The ongoing environmental stresses resulting from the actions of human activities severely affect the physiochemical properties of water and soil which are likely to result in poor water quality, extreme pH gradients and temperatures, hypersalinity, poor soil structure, reduced nutrient compositions, disrupted nutrient cycles, reduced ecological processes such as photosynthesis and decomposition, and increased heavy metal concentrations. This can lead to the deterioration of mangrove habitats, reduced zonation, dispersion and survival rates of mangrove vegetation, decline in seedling propagation and establishment, and impedes the reestablishment and recovery rates of rehabilitated ecological frameworks. As such, the current state of water and soil present within mangrove forests can serve as one indicator of the overall health status of the ecosystem itself. Since soil-water relationships are known to affect the overall ecological balance of many ecosystems, inclusive of mangrove forests, it is imperative to prioritise conservation endeavours aimed at mitigating the pollution of mangrove soil and water to guarantee the continuity of the ecological benefits and services provided by mangrove ecosystems.
ACKNOWLEDGEMENTS
- We thank the staff of the University of Guyana (Department of Biology), The Centre for the Study of Biological Diversity (CSBD), the National Agricultural Research & Extension Institute (NAREI), and the Mid-Atlantic Oil and Gas Company for their guidance and provision of resources to complete this review article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML