-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2022; 12(1): 1-3
doi:10.5923/j.env.20221201.01
Received: May 4, 2022; Accepted: Jul. 1, 2022; Published: Jul. 15, 2022

An Investigation of Endophytes from Roots of Pea Plant
Oluwapelumi Shodubi, Jordan Johnson, Jennifer Laifa, Hattie Spencer
Department of Natural Sciences & Environmental Health, Mississippi Valley State University, Itta Bena, USA
Correspondence to: Jennifer Laifa, Department of Natural Sciences & Environmental Health, Mississippi Valley State University, Itta Bena, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Endophytes are bacteria that are found inside plants protecting the plant and enhancing plant growth. They are found in all species of plants, but their relationship is not well understood. The study hypothesized that the endophytes would be found in the roots of pea plants. In the present study, seeds of the pea were purchased from the garden center and grown for six weeks. After six weeks, the plants were harvested, measured, and weighed. The roots were separated from the shoots and roots were cleaned to remove debris. The roots were then sterilized using sterile water and 70% ethanol. The roots were mixed in magnesium sulfate solution using a mortar and pestle. The tryptic soy agar plates were used to grow bacteria. After 24 hours of incubation, the morphology of the colonies was observed. A simple staining procedure was performed using methylene blue. The Gram staining procedure was also conducted. A catalase test was conducted as well. A starch hydrolysis test was also performed. The colonies were also grown on MacConkey agar. The result indicated that the colonies were smooth, and elevated rod-shaped gram-negative bacteria cells. The result indicated that cells were catalase-positive and could not hydrolyze starch. Although the cells grew on MacConkey, they could not ferment lactose. The strain of bacteria was found to be from the genus Pseudomonas. In conclusion, Pseudomonas is one of the bacteria found in the roots of pea plants.
Keywords: Endophytes, Root, Pseudomonas
Cite this paper: Oluwapelumi Shodubi, Jordan Johnson, Jennifer Laifa, Hattie Spencer, An Investigation of Endophytes from Roots of Pea Plant, World Environment, Vol. 12 No. 1, 2022, pp. 1-3. doi: 10.5923/j.env.20221201.01.
Article Outline
1. Introduction
- Endophytes are bacteria and fungi that live in plants without causing harm. They are present in what is known as the plant rhizosphere. Several authors agree that endophytes are beneficial to plants but differ in how they define them. There are three types of endophytes: obligate, facultative, and passive. Further, Hardoim et al. (2008) [1] define facultative endophytes as bacteria that are alternating their habitats between the host plants and the soil. Their presence is beneficial to the plants as they promote the growth of plants through phytostimulation, biofertilization, and biocontrol [2]. Using an ecological perspective, [3] classified endophytes as systemic or true and non-systemic or transient. In addition to colonizing the host and having a relationship with the host, other aspects such as antibacterial properties against pathogens, and bioremediation of pollutants are displayed during the interaction [4]. To be able to isolate endophytes, the surface-sterilized host plant must be used. According to El-Deeb et al. (2013) [5], organs such as the roots, shoots, flowers, fruits, and seeds, can be used to isolate endophytes after surface sterilization. From their review, Martinez-Klimova et al. (2017) [6] pointed out that there are questions that need to be answered on how endophytes colonize plants as they are said to be specific and can be found colonizing many host plants. The diversity of bacterial endophytes can be explained by many factors such as the geography and the location of both the host and the endophytes. Another factor as mentioned by Chu and Bae (2021) [7] can be growth factors and conditions during cultivation. Most bacterial endophytes have been found by Anyasi and Atagana, (2019) [8] to exist in extreme conditions such as extreme pH conditions. In addition to geography and growth factors, the host influences the diversity of the endophytes. The plant species, age, organ, and tissue type directly influence the endophytes found in it. Hence, this study was conducted to investigate the endophytes found in the roots of pea plants. Only one isolate was further characterized for the present study.
2. Materials and Methods
- Growing and collecting plant materialPisum sativum (pea) seeds (Fig. 1) were grown in pots for six weeks. After six weeks, the plants were harvested. They were cleaned, and the lengths measured. The shoots and the roots were separated, measured, and weighed.
 | Figure 1. Little marvel pea used in the present study |
3. Results
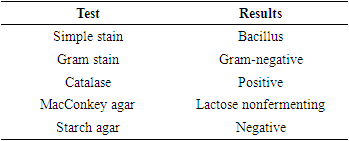
- The results of the present study as displayed in Table 1 indicated that the bacteria were rod-shaped and after carrying the Gram stain procedure, the cells were observed to be gram-negative bacterial cells. The bacterial cells were shown to be catalase-positive. When grown on a MacConkey agar plate the bacterial cells grew but could not ferment lactose. When the starch hydrolysis procedure was carried out on starch agar, the bacterial cells were unable to break down the starch.
|
4. Discussion
- From the present study, Pseudomonas was found to be one of the bacteria colonizing the roots of pea plants. Several authors have identified Pseudomonas as one of the bacteria found in the roots of plants. The ability to degrade organic compounds by Pseudomonas [8] has been attributed to its abundance in host plants [11]. From the study conducted by Pereira et al. (2016) [12] using Lavandula dentata L., Pseudomonas was found to have colonized both roots and shoots. They further claimed that Pseudomonas was responsible for promoting the growth of L. dentata. Pseudomonas was also found by Duan et al. (2013) [13] when isolating endophytes from vascular tissue of Salvia miltiorrhiza roots. They also confirmed the effect of Pseudomonas in promoting growth. They argued that the xylem of the roots was the tissue transporting the growth-promoting materials from the endophytic bacteria to the plant. Walitang et al. (2017) [14] when using seeds of rice found out that Pseudomonas was one of the endophytes with high production of siderophores. Siderophores are small iron-binding molecules that are produced when levels of iron are low. The same positive effect of endophytes was observed by Tyc et al. (2020) [15] when using seeds of wild cabbage revealed that the early development and growth of seeds were due to the endophytes. Pseudomonas, which is found to be one of the most dominant endophytes mentioned in the literature [16], belongs to the phylum Proteobacteria and class gammaproteobacteria [17]. Pseudomonas is one of the genera that is found in the soil, therefore, the openings or cracks from the roots are assumed to be the sites that can be used for colonization by bacteria. Hardoim et al. (2008) [1] refer to Pseudomonas as competent endophytes. Although the endophytes are beneficial to the host, there are cases where they can produce compounds that are toxic as in Pseudomonas producing cyanide which can end up in the host plants and ultimately humans [18].
5. Conclusions
- Because of the need for more food, high good quality vegetables must be produced. Fertilizers are used to grow plants but in the long run, the same fertilizers contaminate drinking water. Researchers have been looking for alternate methods to grow plants using cost-effective practices which are also environmentally friendly. Endophytes seem to be promising to be used as growth promoters in plants. Pseudomonas is one of the bacteria found in the roots of peas. A further study needs to be carried out to investigate the effect of Pseudomonas as growth-promoting bacteria.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge the Department of Natural Sciences and Environmental Health at Mississippi Valley State University where the study was conducted. Funding for the study was provided by Minority Science and Engineering Improvement Program (MSEIP) P120A170085, STEM CHANGERS Mississippi Valley State University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML