-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2020; 10(1): 16-26
doi:10.5923/j.env.20201001.03

Evaluation of Heavy Metal Concentrations and Proximate Compositions of Amaranthus spinosus L. and Talinum triangulare J. and Soils Collected from Dumpsites in Some Selected Areas in Lagos State, Nigeria
Oluwole Surukite O., Makinde Sunday C. O., Ogun Mautin L., Nwachukwu Ifeanyi R.
Department of Botany, Lagos State University, Ojo, Nigeria
Correspondence to: Oluwole Surukite O., Department of Botany, Lagos State University, Ojo, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Anthropogenic activities usually results in pollution, especially in developing countries where low-end waste management is the common practice. Thus, this research evaluates the concentration of heavy metals (Mn, Cu, Zn Pb, and Cd) and proximate compositions of Amaranthus spinosus and Talinum triangulare. Soils were collected in three dumpsites from three different Local Government areas (Alimosho, Amuwo-Odofin and Ojo) in Lagos State, Nigeria. The sampled vegetables and soils were determined using Standard techniques and atomic absorption spectrophotometer for the metal concentration analysis. The level of each metal concentration to the dumpsites was compared to World Health Standard. The results revealed that the two vegetables sampled contained appreciable quantities of nutrients needed for normal human development. Also, the heavy metal analysis shows the concentrations ranges from Mn (0.034-709mg/Kg), Cu (1.624-3.637mg/Kg), Zn (0.671-1.256mg/Kg), Pb (0.000-0.007mg/Kg) and Cd (0.000-0.007) in Amaranthus spinosus; Mn (0.336-0.073mg/Kg), Cu (0.206-1.834mg/Kg), Zn (0.006-0.680mg/Kg), Pb (0.000-0.003mg/ Kg) and Cd (0.000-0.002mg/ Kg) in Talinum triangulare; and Mn (0.000-1.300 mg/Kg), Cu (1.348-1.732 mg/Kg), Zn (0.149-1.100mg/Kg), Pb (0.000-0.008mg/Kg) and Cd (0.000-0.002mg/Kg) in the soil samples of the three dumpsites. However, Amaranthus spinosus, Talinum triangulare and soils of most of these dumpsites accumulated minimal concentrations of these metals. In fact Pb and Cd were not detected in some vegetables and soils. All the concentrations of metals studied in soil and vegetables were found to be lower than the maximum permissible limit of heavy metal in soil and vegetable stated by the World Health Organization (WHO) which implies that the vegetables are presently safe for human consumption. Thus, the higher concentration of metals in wastes of dumpsites and their vegetables, the increased threat it posed to the consumers. Hence, it therefore recommends that consumption of Amaranthus spinosus, Talinum triangulare and other vegetables from dumpsites should be avoided.
Keywords: Heavy metals, Proximate composition, Amaranthus spinosus, Talinum triangulare, Dumpsite, Soil
Cite this paper: Oluwole Surukite O., Makinde Sunday C. O., Ogun Mautin L., Nwachukwu Ifeanyi R., Evaluation of Heavy Metal Concentrations and Proximate Compositions of Amaranthus spinosus L. and Talinum triangulare J. and Soils Collected from Dumpsites in Some Selected Areas in Lagos State, Nigeria, World Environment, Vol. 10 No. 1, 2020, pp. 16-26. doi: 10.5923/j.env.20201001.03.
Article Outline
1. Introduction
- The contamination of soil and vegetables by heavy metals is a global environmental issue. Heavy metals are generally referred to as those metals that possess a specific density of more than 5 g/cm3 and adversely affect the environment and living organisms [1]. Heavy metals, being persistent and non-biodegradable, can neither be removed by normal cropping nor easily leached by rain water [2]. They might be transported from soil to ground waters or may be taken up by plants, including agricultural crops. For this reason, the knowledge of metal-plant interactions is also important for the safety of the environment [3]. Source of anthropogenic contamination include the addition of manures, sewage sludge, fertilizers and pesticides to soils. Several studies has identified the risks in relation to increased soil metal concentration and consequent plant uptake [4,5]. Analysis of vegetables grown in locations close to industries in Greece has been reported to elevate the levels of heavy metals contamination [6,7,8]. The results of the study indicated significantly higher levels of metal accumulation in leafy vegetables as compared with root vegetables. However, some micro-elements are toxic to both animals and plants at high concentrations. For instance, copper (Cu), zinc (Zn) arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) are toxic even at small concentrations [3]. These metals can pose a significant health risk to humans, particularly in elevated concentrations above the very low body requirements [8]. So, the metals must be controlled in food sources in order to assure public health safety [9]. Excessive amount of heavy metals in food cause a number of diseases, especially cardiovascular, renal, neurological, and bone diseases [10]. These metals could reach food chain through various biochemical processes and ultimately biomagnified in various trophic levels and eventually threaten the health of human. There has been increasing interest in determining heavy metal levels in public food supplied. However, their concentration in bio-available form is not necessarily proportional to the total concentration of the metal [11,12]. The quality of ecosystem becomes altered, when heavy metals find their way, somehow, into it through human and natural activities. These activities are one of the most pressing concerns of urbanization in developing countries like Nigeria, which result in the problem of solid, liquid and toxic waste management. Such waste may be toxic or radioactive nature [13,14]. Such waste management problems include heaps of uncontrolled garbage, roadsides littered with refuse, streams blocked with rubbish, prevalence of automobile workshops and service stations, inappropriately disposed toxic waste and disposal sites that constitute a health hazard to residential areas [15,16,17].Lagos State is a Metropolitan city with a good number of industries of various categories, markets, and trades. These anthropogenic activities usually result in pollution, especially in developing countries where low-end waste management is the common practice [18]. Large amounts of wastes are generated and dumped daily [19,20]. There is a serious environmental concern, considering that a larger fraction of the wastes is hazardous in nature [21]. The population increase and the rapid growth of industrial processes particularly in major cities have led to the birth of new world and thus have greater impact on human and the environment. The expansion of industrialization have given birth to environmental pollution and the greater volume of industrial chemical discharge has added to the growing pack of untreated domestic waste which contains heavy metals. The evacuation of domestic, commercial and industrial garbage which may contain toxic materials such as Pb, Cu, Cd, Hg, Mn, and Zn from batteries, insecticides, nail-polish, cleaners, polyvinyl chloride made containers, pesticides and other various products in the world is a predicament that continues to grow with human development [22,23,24].The disposal of materials that contains heavy metals in open dumpsites are of concern and pose dangers to people in contact with the soil and plants of these sites in which they are disposed. In Nigeria, leachates from refuse dumpsites constitute a source of heavy metal pollution to both soil and aquatic environments [25,26]. In some cases, wastes are dumped recklessly with no regards to the environmental implications, while in some dumpsites, wastes are burnt in the open and ashes abandoned at the sites. The concentrations of heavy metals in soil around waste dumpsites are influenced by types of wastes, topography, runoff and level of scavenging [27,28]. Once heavy metals are deposited in the soil, they are not degraded and persist in the environment for a long time causing serious environmental pollution [29]. There is a growing concern about the possibility of soil contamination resulting in the introduction of elements in food chains through uptake by plants and thereby affecting food safety [30]. They accumulate in soil and plants having a negative influence on physiological activities of plants such as photosynthesis, gaseous exchange and nutrient absorption which result in plant growth reduction and dry matter accumulation [31]. Although the rate of metal uptake by plants could be influenced by factors such as metal species, plants species and so on [32]. However, Water leaf (Talinum triangulare J.) is a cosmopolitan weed belonging to the family Taliniaceae and commonly found in the humid tropics. It has been recorded for several countries in West and Central Africa [33,34]. Water leaf is eaten as a vegetable throughout the tropics including many countries in West and Central Africa. Talinum triangulare leaves are used in the preparations of slightly slimy soups and stews to compliment the starchy main dishes [33,34]. In southern Nigeria, where is called ‘Gbure’, it is commonly mixed with Jute mallow (Chochorus olitorius). South American used water leaf in treating some ailments such as contusions, inflammations and tumors. Decoctions are used for painful eyes and aid recovery of blows and falls [35]. More so, a phytochemical analysis on Talinum triangulare revealed that it contains some vital phytochemical used for medicinal purposes. These phytochemical include tannins, alkaloids, saponins, and flavonoids, which suggests its potential medicinal and dietary benefits [33]. Amaranthus spinosus L. is both annual and perennial herb belongs to family Amaranthaceaea, with common name (Spiny amaranth, prickly amaranth, spiny pigweed (English). It is commonly or widely distributed within tropical and subtropical regions of Africa, Southeast Asia and USA. In Nigeria especially Yoruba community all species are referred to as “tete” even though they may add a second name to indicate a particular variety or species. Amaranthus spinosus is widely used because of it medicinal, nutritional and otherwise properties; which includes treatment of gonorrhea; an emmenagogue, emollient and antipyretic; applied externally to treat eczema, burns, wounds, boils, earache, haemorroids and so on [36,37,38]. Thus, Picking of vegetables like Talinum triangulare (waterleaf) and Amaranthus spinosus growing naturally on non-agricultural land or sourcing it from the contaminated environment may pre-dispose humans to toxic pollutants. In addition, Waterleaf and Amaranthus propagates prolifically; it has extensively produced adventitious roots which have the potential to absorb toxic substances from aqueous media [39]. It is on this basis that this study was designed to evaluate the concentrations of heavy metals in both soils and leafy vegetables from some selected dumpsites in Lagos state, Nigeria.
2. Materials and Methods
- Collection of Plant MaterialsThe soils and two vegetables (Talinum triagulare and Amaranthus spinosus) (Figures 2 and 3) were collected from dumpsites located in three different Local government areas in Lagos State, Namely; Alakija Dumpsite in Amuwo-Odofin Local Government area, Ketu-Ijanikin Dumpsite in Ojo Local Government area and Agbado-Oke-Odo Dumpsite in Alimosho Local Government area (Figure 1). The soils were collected at a depth of about 15cm around the plants’ roots.
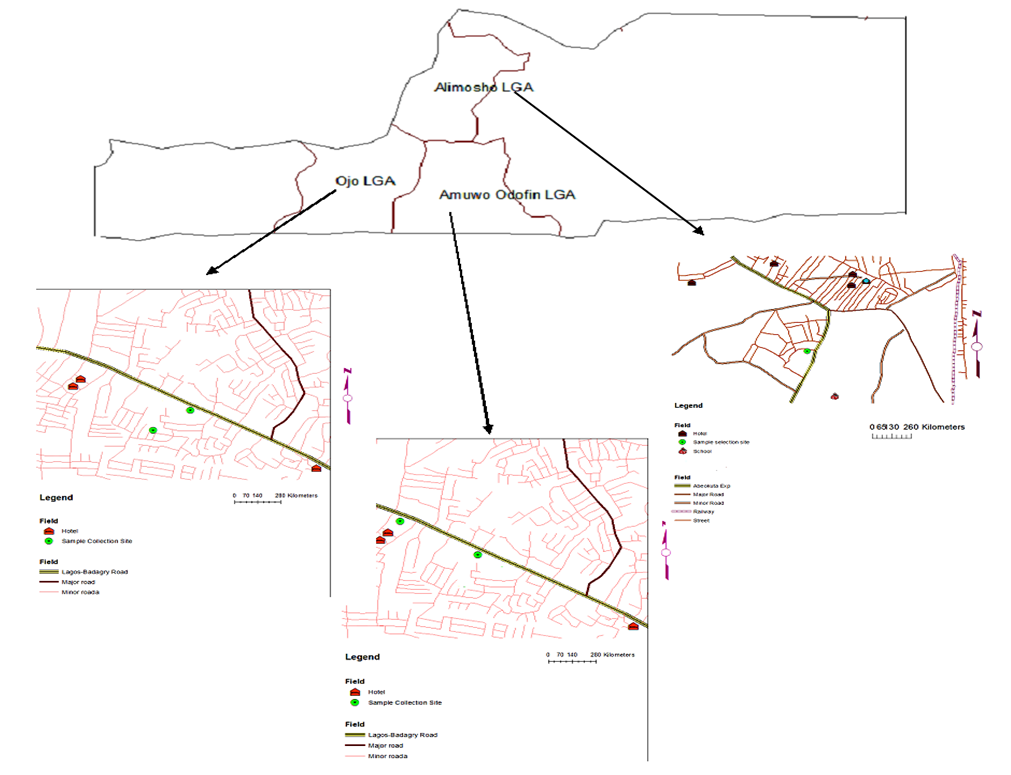 | Figure 1. Map of Lagos State showing the sample locations |
 | Figure 2. Image showing Amaranthus spinosus |
 | Figure 3. Image showing Talinum triangulare |
3. Results and Discussion
3.1. Results
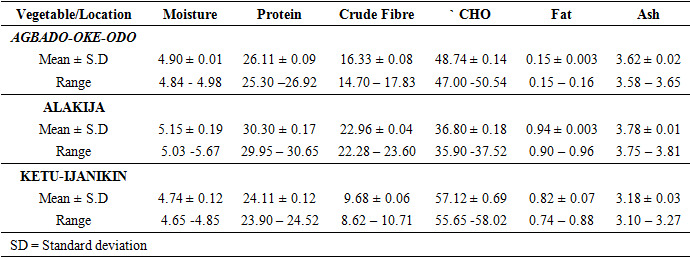
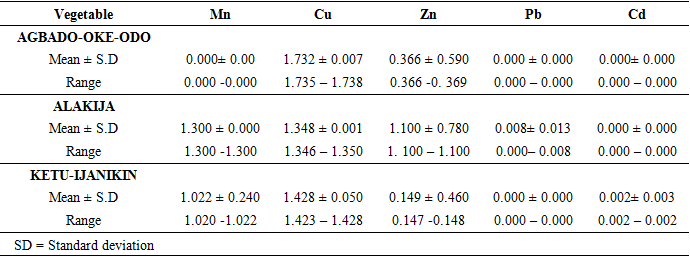
- Proximate Analysis of Amaranthus spinosus in different dumpsitesTable 1 shows the proximate composition of Amaranthus spinosus in the three dumpsites. Thus, the result revealed that the Amaranthus spinosus from Alakija dumpiste has the highest percentage moisture contents while, Agbodo-Oke-odo and Ketu-Ijanikin had similar moisture contents (Figure 4). A. spinosus for Alakija had the highest protein content followed by Agbodo-Oke-Odo while the least values were recorded in Ketu-Ijanikin (Table 1, Figure 4). The crude fibre of A. spinosus in Alakija has the highest mean values followed by those in Agbado-Oke-Odo while Ketu-Ijanikin having the least crude fibre contents (Table 1, Figure 4). The crude carbohydrate content of A. spinosus found in Ketu-Ijanikin has the highest values followed Agbado-Oke-Odo with Alakija having the least crude carbohydrate contents (Table 1, Figure 4). The crude fat contents of A. spinosus in both Alakija and Ketu-Ijanikin showed similar values while Agbado-Oke-Odo has the least values. The ash contents of A. spinosus in both Agbado-Oke-Odo and Alakija have similar values while Ketu-Ijanikin contains the least values of Ash contents (Table 1, Figure 4).
|
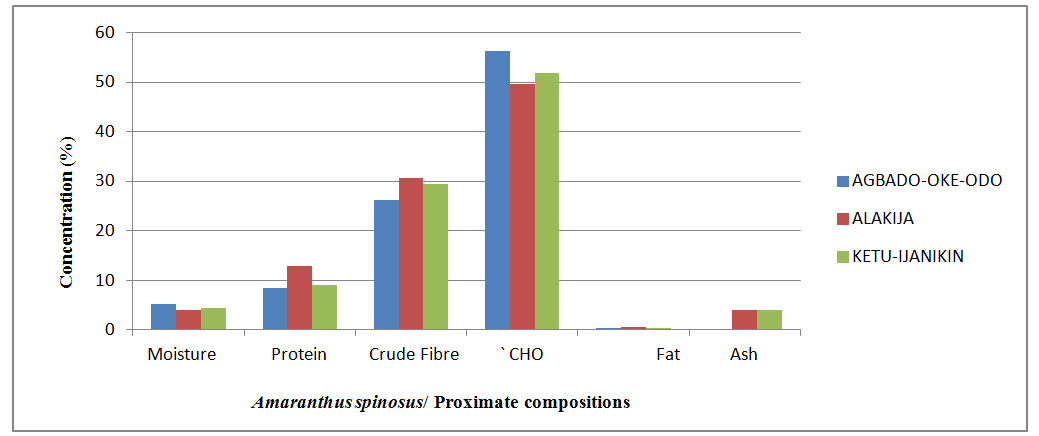 | Figure 4. Comparison of the proximate compositions of Amaranthus spinosus collected from different locations |
|
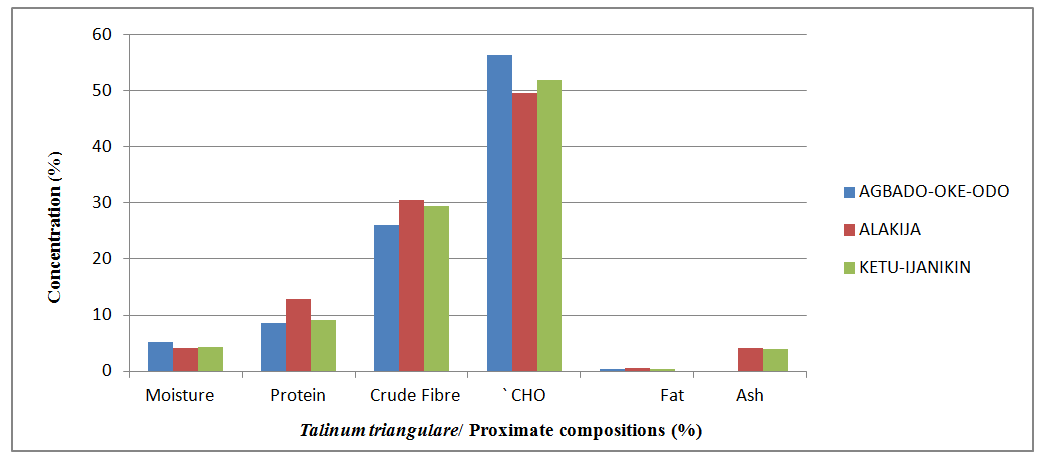 | Figure 5. Comparison of the proximate compositions of Talinum triangulare collected from different locations |
|
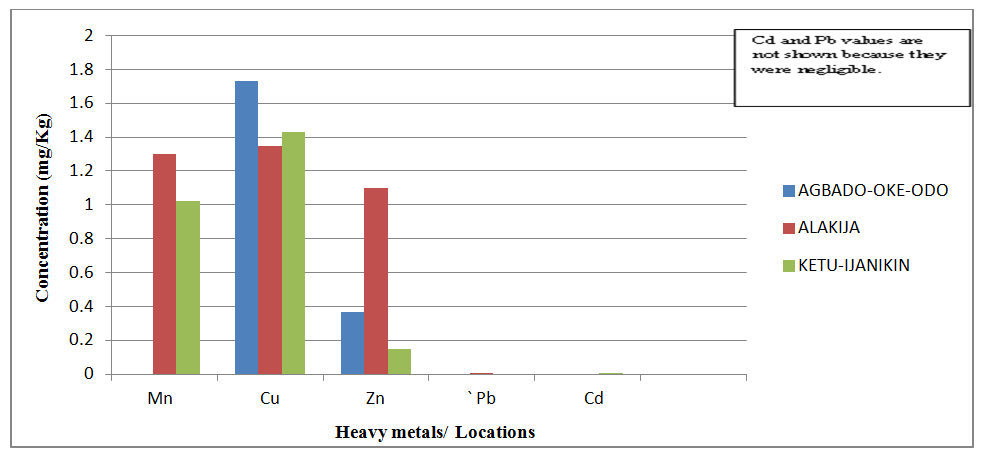 | Figure 6. Comparison of Heavy Metal concentrations in Amaranthus spinosus from three different dumpsites |
|
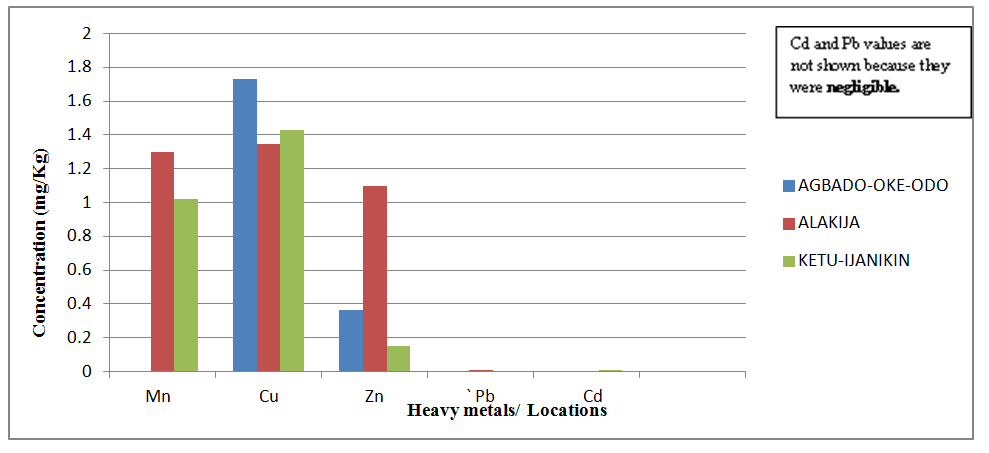 | Figure 7. Comparison of Heavy Metal concentrations in Talinum triangulare from three different dumpsites |
|
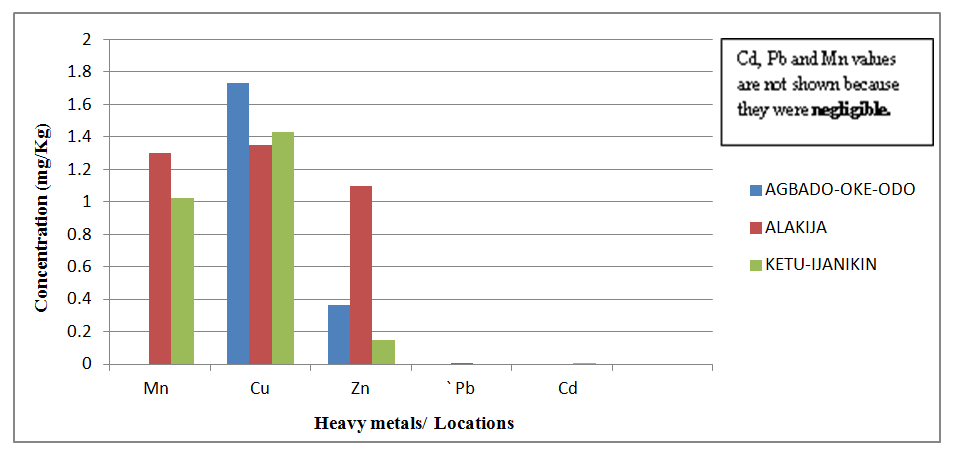 | Figure 8. Comparison of Heavy Metal concentrations in Soil collected from three different dumpsites |
3.2. Discussion
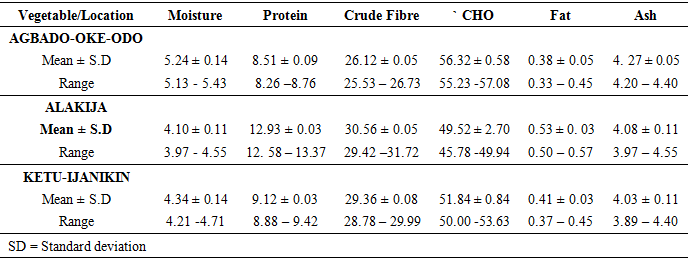
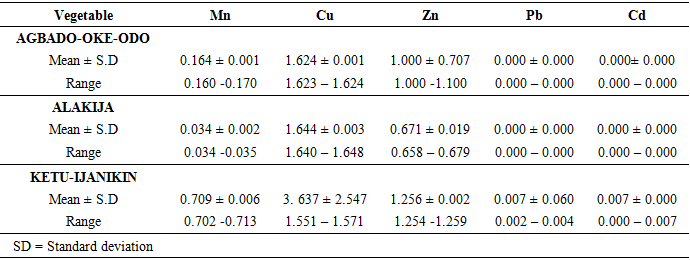
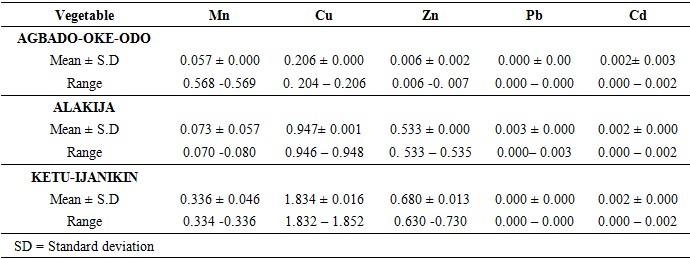
- Proximate Analysis of Amaranthus spinosus and Talinum triangulareThe proximate analyses of the two leafy vegetables obtained from the three different dumpsites are shown in Tables 1 and 2. The percentage moisture contents of Amaranthus spinosus and Talinum triangulare varied from 4.74% to 5.24% in the two leafy vegetables from the three locations. These values were lower than those reported earlier for some Nigerian leafy vegetables by Onwordi et al. [43]. The variations in the moisture contents based on locations were supported by Oluwole et al. [33,44]. This was attributed to differences in soil composition and or soil characteristics such as soil water content and soon. However, the moderate moisture content provides for increased activity of water soluble enzymes and co-enzymes needed for metabolism of these two vegetables [45]. The crude protein content varied from 24.11-30.30% in Amaranthus spinosus (Table 1) and between 8.51-12.93% Talinum triangulare (Table 2) between three locations. This result was lower than what was reported by Onwordi et al.[43], Fagboun et al. [46] and Asaolu et al. [47] in some vegetables such as Bitter leaf (50.64%), Indian Spinach (58.80%), Bush-buck (66.60), Scent leaf (62.71%), Amaranthus hydridus (49.02%), Hibiscus sabdariffa (46.56%), Telfairia occidentalis (61.70%). But the result of this finding was higher than the findings of Oluwole et al. [44]. Thus, plant foods that supply the body with more than 12% of their caloric value from protein have been reported to be good source of protein [48]. This shows that the two leafy vegetables are rich source of protein. The crude fibre content ranged from 9.68-16.33% in Amaranthus spinosus (Table 1) and 26.12-29.36% in Talinum triangulare (Table 2). This fell within the reported range values (8.50-20.90%) for some Nigerian Vegetables [49]. Dietary fibre helps to lower serum cholesterol level, risk of coronary heart diseases, constipation and diabetes [50]. The carbohydrate ranged from 36.80-57.12% in Amaranthus spinosus (Table 1) and 49.52-56.32% in Talinum triangulare (Table 2). The value obtained for carbohydrates was above the range of 30.05% as reported by Sanni and Olaofe [51]. Also, the carbohydrate content of A. cruentus (30.10%) could be comparable to 29.4%, 31.34% and 32.84% of A. cruentus, Cochorus olitorius and A. argenta respectively as reported by Onwordi et. al. [43] but the value was higher than the values reported for some leafy vegetables consumed in Nigeria which includes Vernonia amygdalina (8.65%), O. gratissimum (1.22%) and Hibiscus sabdarifa (15.79%) reported by Asaolu et al., (2012); and Oluwole et al. [44] for Ocimum gratissimum and Amaranthus cruentus. Carbohydrate constitutes a major class of naturally occurring organic compounds which are essential for the maintenance of life and also provide raw materials for many industries [52]. Also, it was above the values reported by Oluwole et al. [44] The percentage fat content ranged from 0.15-0.94% in Amaranthus spinosus (Table 1) and 0.38-0.53% in Talinum triangulare (Table 2). These values were lower compared to those in some vegetable by Asaolu et al. [47] and Oluwole et al. [44] such as A. cruentus and O. gratissimum are 1.62% and 3.59% respectively. The percentage ash content ranged from 3.18-3.75% in Amaranthus spinosus across the three locations (Table 1) and 4.03-4.27% in Talinum triangulare across the three locations (Table 2). Thus, the ash content was higher in Talinum triangulare than those of Amaranthus spinosus across three locations (Tables 1 and 2). Thus, the ash content obtained are lower to those of O. gratissimum reported by Fagboun et al., [46] and A. hybridus [45] and also there was lower ash contents of A. cruentus (30.88%) and O. gratissimum (7.12%) respectively as reported Oluwole et al. [44]. Ash content is of significant importance in foods as they account for the mineral constituents [53]. However, various differences were observed in the proximate compositions of Amaranthus spinosus and Talinum triangulare across the three locations. These differences have been reported by Oluwole et al. [33,44], they attributed these variation to abiotic factors such as water, soil mineralization, and so on within and around the locations.Heavy Metal Concentrations of Plants and Soil Samples UsedThe concentration of heavy metals in plants and soil samples are presented in Tables 3-5. Results above showed that Manganese (Mn) obtained from Amaranthus spinosus in Ketu-Ijanikin is higher than others, whereas the quantities Mn found in three areas sampled were lower than the World Health Organization (W.H.O) of 5000mg/Kg [54] (Table 3). This result was similar to the findings of Adaikpoh [55] who found similar results on distribution and enrichment of heavy metals in soils from waste dumpsites within Imoru and environments, Southwest Nigeria. Also, the concentrations of Mn obtained in Talinum triangulare in three areas were lower than the W.H.O standards (Table 4). More so, the concentrations of Mn found in the soils of the sampled locations were between the permissible limits of W.H.O, indicating safety of the soils. Thus, all the concentration of manganese values was found to be lower than the World Health Organization standard for manganese in soil. This is because Manganese is found naturally in the most soils as it is one of the most essential minerals for life [56].The concentrations of Copper (Cu) found in Amaranthus spinosus collected from Ketu-Ijanikin (3.637mg/ Kg) were higher than those collected from Alakija (1.644mg/Kg) and Agbado-Oke-Odo (1624) respectively (Table 3). Thus, the results of Cu collected from three dumpsites were lower than W.H.O limits of 5.0-20.0 mg/Kg [54,57]. Also, the Cu concentrations found in Talinum triangulare from Ketu-Ijanikin (1.834mg/Kg) were higher than other two collected from Alakija (0.947mg/Kg) and Agbado-Oke-Odo (0.206 mg/Kg) respectively (Table 4). The results from Ketu-Ijanikin three dumpsites were lower than W.H.O limits of 5.0-20.0 mg/Kg [57]. More so, the concentration of Cu found in the soils of the three dumpsites ranging from 0.206-1.834mg/Kg were lower compared to W.H.O standard limits of 5.0-20.0 mg/Kg [57]. These results agree with work of Ogundele et al. [23], when they undergo the assessment of heavy metal pollution of waste dumpsites in Kwara State using Carica papaya, Musa spp. and Corchorus olitorous as Indicators. The soil showed heavy metal contents below the threshold values reported in literature for agricultural soils, therefore establishing the suitability of the soil for planting [58]. Also, there are a few studies which also demonstrated contamination of forage plants by human waste accumulation activities.The concentration of Zinc (Zn) found Amaranthus spinosus collected from Ketu-Ijanikin and Agbado-Oke-Odo was higher than those of Alakija (Table 3). This result is lower than W.H.O standard limits of 10-150 mg/kg as reported Boularbah et al. [57]. This finding also agreed with work of Ogundele et al. [23], although, the Zn levels in the plants were lower than the recommended limits of 60.0 mg/kg in normal plant; hence, there is need to monitor the accumulation level. Also, concentration of Zinc (Zn) found Talinum triangulare collected from Ketu-Ijanikin and Alakija was similar and higher than those of Agbado-Oke-Odo (Table 4). This result is lower than W.H.O standard limits of 10-150 mg/Kg as reported Boularbah et al. [57]. It also, concurred with findings of Ogundele et al. [23,24], it reported that although, the Zn levels in the plants were lower than the recommended limits of 60.0 mg/Kg in normal plant, there is need to monitor the accumulation level. More so, the concentration of Zinc (Zn) found in the soils collected from Alakija were higher than those of Ketu-Ijanikin and Agbado-Oke-Odo (Table 5). This result is lower than W.H.O standard limits of 10-150 mg/Kg as reported Boularbah et al. [57] The concentration of Lead (Pb) and Cadmium (Cd) were not detected in samples Amaranthus spinosus collected from Alakija and Agbado-Oke-Odo but those found in the Amaranthus spinosus collected Ketu-Ijanikin (Table 3) were lower than W.H.O standard limits of 100mg/Kg and 3mg/Kg respectively as reported by Ilori et al. [54]. Ilori et al. [54] findings also confirmed the finding of this study when they reported similar results in their work titled: Investigation of heavy metal content on dumpsites soil and vegetables grown: a case study of Ilesha metropolis, Nigeria. Also, the concentrations of Lead (Pb) were not detected in samples of Talinum triangulare collected from Ketu-Ijanikin and Agbado-Oke-Odo (Table 4) but those found in the Talinum triangulare collected Alakija were lower than W.H.O standard limits of 100mg/Kg as reported by Ilori et al. [54]. Whereas, Cadmium (Cd) (0.002mg/Kg) was discovered in all the samples of Talinum triangulare collected from different locations studied. However, this result was lower than the W.H.O standard of 3mg/Kg as reported [54]. More so, Pb were not detected in the soils collected Agbado-Oke-Odo and Ketu-Ijanikin respectively but was detected in the soil of Alakija (Table 5). The detected concentration was within the permissible limits of W.H.O as reported by Ilori et al. [54]. Whereas, Cd was not detected in the soil samples of Agbado-Oke-Odo and Alakija while the concentration found in the soil of Ketu-Ijanikin were within the limits of W.H.O. [54].Moreover, one or more of these controlling factors may have been responsible for the wide variation in the concentration and pattern of uptake observed for the various metals in the plants and soils. The measure of concentration of these metals in the vegetables reflected their corresponding concentrations in the soil. Thus, agreed with what some authors have noted, that plant uptake of metal from soil is largely determined by the concentration of the metal in the soil matrix [59]. Manganese had low significant concentration compared to W.H.O standard which means there is a likelihood of anthropogenic input on the vegetables and soils from the dumpsites.
4. Conclusions
- This research has shown that there is heavy metal pollution on dumpsite soils which are as result of the effect of heavy metal containing wastes that are improperly disposed there. Thus, buildups of heavy metal on these dumpsites are capable of being leached to the nearest place of uptake by vegetables and other plants around them. Although, the soil and vegetable studied were found to be safe for now but there are concerns for the gradual and continuous buildup which has started and indicated in the obvious differences of the dumpsites’ metal concentrations. This study showed that continuous harvesting and cultivation of consumable vegetables in or around dumpsites is highly risky to human and also it is necessary to take measures to stop harvesting and cultivating vegetables for human consumption.From the findings of this research, it is therefore recommended that despite the richness of the proximate compositions of Amaranthus spinosus and Talinum triangulare collected from the three different dumpsites, heavy metals concentrations present are capable of posing threats to humans health as a result of it bioaccumulation properties. Thus, vegetables from these dumpsites should be avoided
ACKNOWLEDGEMENTS
- Authors extend gratitude to All members staff in Department of Botany, Lagos State University, Ojo, Nigeria for their scholarly advise during the research and compilation of the manuscript, and lastly to our anonymous reviewers for their editorial insights.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML