-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2020; 10(1): 1-9
doi:10.5923/j.env.20201001.01

Using Cilantro (Coriandrum Sativum) to Remove Cadmium from Contaminated Water
Njue Martin, Mbuvi Harun, Margret Nga’nga’, Mbugua Gerald
Department of Chemistry, Kenyatta University, Nairobi, Kenya
Correspondence to: Mbuvi Harun, Department of Chemistry, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Accessibility to safe drinking water poses great risks to human health since heavy metal ions can accumulate their amounts along the food chain. Methods already in use for cleaning contaminated water have resulted in generating toxic sludge and are expensive. Cilantro leaves has been used to reduce the contamination of heavy metals like lead, mercury and copper in rod shellfish as contains bioactive components of flavonoids, saponines and fibers which are capable of absorbing heavy metal ions by chelating action. However, the most effective part of cilantro and the most effective form i.e. when green or dry has not been reported. Consequently, the present study aimed at investigating adsorption of Cd2+ ions by fresh and dry cilantro leaves and stems from simulated water. Cilantro fresh leaves and fresh stems were grounded to obtain the wet adsorbents while for the dry adsorbents, fresh cilantro leaves and stems were sun dried at room temperature until their weighed dry masses remained constant and grounded to fine powder. Batch sorption studies were carried out while varying parameters of contact time, shaking speed, temperature, adsorbent dose, pH and initial concentration of metal ions in solution. Residue Cd2+ ions concentrations were determined using atomic adsorption spectroscopy (AAS). The adsorption of Cd2+ ions was described by Langmuir isotherm with Qmax values of 250.00, 51.28, 4.68 and 15.06 mg/g for fresh leaves (FL), fresh stems (FS), dry leaves (DL) and dry stems (DS) biomasses respectively. The results from this study suggest that fresh and dry cilantro leaves and stems are potential adsorbents of Cd2+ ions.
Keywords: Cilantro, Cadmium, Adsorption capacity
Cite this paper: Njue Martin, Mbuvi Harun, Margret Nga’nga’, Mbugua Gerald, Using Cilantro (Coriandrum Sativum) to Remove Cadmium from Contaminated Water, World Environment, Vol. 10 No. 1, 2020, pp. 1-9. doi: 10.5923/j.env.20201001.01.
1. Introduction
- The world population is ever-increasing causing rapidly industrialization leading to more demand for the dwindling water resources, making it precious in many more countries. Indeed, a new report from the World Bank indicates that water pollution threatens nearly all the globally agreed sustainable development goals (World Bank Group, 2019). For many decades, the problem of water shortage has proved to be a serious issue affecting Kenya caused by years of repeated droughts, poor water supplies management, pollution of water and a huge increase in demand as the population increase (Marshall, 2011). Majority of the metals ions bio-accumulate and hence becoming a threat to human life. The direct deposition of heavy metals ions invested waste in water bodies needs to be checked to minimize the environmental degradation (Khan et al., 2008). Some of the heavy metals ions include: nickel, cerium, iron, uranium, bismuth, antimony, chromium, gold, copper, cobalt, gallium, zinc, platinum, uranium, thallium, tin, tellurium, vanadium and manganese (Fernández-luqueño et al., 2013). The introduction of vast quantities of wastewater contaminated with heavy metal ions which when ingested above the permitted level, they may cause severe health problems (Barakat, 2011). Heavy metals soluble compounds in aqueous water bodies is a major problem because of their harmful nature on the living things in the water bodies (Gupta and Rastogi, 2008). Cadmium, which is used in battery manufacture, paints, plastic industry and electroplating is a known toxic metal. All heavy metals occur in surface waters and in particulate, dissolved phases as well as in colloidal, though dissolved concentrations are usually low. The heavy metal compounds solubility in surface waters is controlled by the water pH, the type and amount of ligands on which the metal could adsorb the redox environment of the system and the oxidation state of the mineral constituents (Kennish, 1992).Developing research on a different treatment methods for example; membrane processes, coagulation, precipitation, sedimentation, filtration, flotation, biological methods, chemical processes, electrochemical method ion exchange and adsorption; with various degree of successes leads to elevated dramatic outcomes in the scientific world. However, these methods are expensive for use in developing countries, especially in rural areas. Consequently, there has been an urgent need to find modern technologies or bio-materials to rid heavy metal ions from contaminated water and biosorption is a promising option (Gupta and Rastogi, 2008). Biosorption uses biological materials capability to hold metal ions in contaminated water by either physicochemical or metabolically associated pathways for the uptake (Gupta and Rastogi, 2008). The major benefits of biosorption as opposed to conventional methods are that it is cheap and efficient, possible regeneration of sorbent, no additional nutrients is required, and possible metal recovery (Zhao et al., 2011). Some biosorption studies use special isolated strains, locally available biomass while others use processed raw materials to increase their biosorption ability (Volesky and Holan, 1995). Large amount of waste substances of organic origin such as dead leaves, seed shells, barks, sawdust, roots and oil cakes from numerous plants in their powder form have found their use in the elimination of heavy metals ions.Cilantro (Coriandrum sativum) mostly refered as Coriander or dhania is an annual plant of parsley family (Apiaceae) within order Aprales, is natural to the Mediterranean area and is widely grown in Russia, India, Central Europe, Bangladesh, and Morocco (Nazrul et al., 2009). Cilantro has both medicinal and nutritional properties. It contains several active ingredients, primarily monoterpenes, limpnene, α-pinene, pcymene, γ-terpinene, citronellol, borneol, camphor, coriandrin, geraniol, dihydrocoriandrin, flavonoids coriandrons A-E, and essential oils. Cilantro has been shown to have many pharmacological effects like antifertility, digestive stimulant antihyperlipidmic, antihyperglycemic, antioxidant, hypotensive and antiproliferative. Coriander is also utilized in detox diet (Leena et al., 2012). Cilantro can be cultivated commercially or in small scale in kitchen gardens, flower pots and planting bags within homesteads. It is readily available, inexpensive and has shown promise in eradicating certain metals ions, for instance copper, mercury and lead that can be dangerous to human health (Ravichandran, 2011). In another study, several natural substances were found to chelate given heavy metal ions while others did not. However, only one compound-HMD (Heavy Metal Detox)-tested in double blind, placebo controlled tests was found to be effective in mobilizing and excreting metals in the faeces and urine for all the metals experimented. HMD consists of a homeopathic homaccord of cell decimated Chlorella, (Cilantro) Coriandrum sativum leaf tincture and Chlorella Growth Factor was found to be a synergistic powerful chelating formula which was cost effective (George, 2005). Therefore the study aims at investigating on the ability of cilantro biomass efficiency and capacity to remove cadmium metal ions dissolved in water through adsorption.Adsorption is a surface process in which multi-component fluids are attached to the surface(s) of a solid adsorbent(s), forming chemical or physical bonds. It is being recognized as one of the widely applied and most efficient fundamental approach in contaminated water treatment methods which mainly revolves on its technical feasibility, economical viability, socially acceptable and simplicity (Foo and Hameed, 2010). The interaction between some metal ions adsorbed onto the adsorbents surfaces and the metal ions concentration in the solution at equilibrium conditions can be expressed by adsorption isotherms (Reddy et al., 2014). There are many isotherm equations for adsorption but the two mainly applied are the Freundlich and Langmuir isotherms (Katircioğlu et al., 2008). Langmuir isotherm represents equilibrium distribution of adsorbate between the liquid and solid phases. The model is applicable for the monolayer sorption onto a surface which contains a fixed number of equal sites. The Langmuir isotherm assumes a uniform dynamism of adsorption onto the surface and no migration of adsorbate on the plane of the surface (State and State, 2012). The model views every adsorption site as energetically equivalent and identical i.e. thermodynamically, every location can hold only one adsorbate molecule (Al-anber, 2011). The linear form of Langmuir adsorption isotherm is shown by the given equation (Katircioğlu et al., 2008);
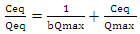 | (1) |
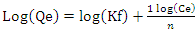 | (2) |
2. Materials and Methods
- Cilantro samples were bought at Githurai market in Kiambu County where there is continuous supply throughout the year. The sample was identified by a plant taxonomist at plant science department in Kenyatta University. The sample was deposited at Plant Science Department Hebanum at Kenyatta University. The biomass was taken to Kenyatta University chemistry laboratory where it was thoroughly washed using distilled water to get rid of soil and dust particles. For the wet biomass, the fresh leaves and stems were chopped 1cm and then grounded to increase the surface area for adsorption. For the dry biomass, fresh cilantro stems and leaves were sun dried at room temperature until the weighed dry masses remained constant to ensure that negligible moisture content was in the samples. The dry samples were then cut and grounded to fine powder. The moisture content was calculated by the difference between the wet and dry sample masses. Chemicals and Reagents: Analytical grade Cd(NO3)2.4H2O of molecular mass 308.47 with 99.0% purity salts obtained from Sigma Aldrich Company was used for the preparation of the stock solutions of Cd2+ ions. Stock solution containing 1000 mg/L of Cd2+ ions was made by diluting 2.754 g Cd(NO3)2 in 1000 mL of distilled water in 1000 mL volumetric flask. A working solution of 100 mg/L of Cd2+ ions was prepared by diluting 100 mL of Cd2+ ions of stock solution to 1000 mL using distilled water in a 1000 mL volumetric flask. Standard solution of 2, 4, 6, 8 and 10 mg/L of Cd2+ ions was prepared by diluting 2, 4, 6, 8 and 10 mL respectively of the 100 mg/L working solution in a 100 mL volumetric flask using distilled water. 1.0 M of Standard HCl acid with 71.0% purity was prepared by diluting 68 mL of the acid and diluting it to 1000 mL with distilled water. 1.0 M NaOH base solution of molecular weight 40 from Merck Limited (India) was prepared by diluting 40 g of NaOH salt in 1000 mL distilled water. 1.0 M of NaOH and 1.0 M of HCl were used for pH adjustments during the batch adsorption studies. Instrumentation: Residue Cd2+ ions concentration in the various solutions were determined using AAS (Buck Scientific 210 HZ 50-60; USA), at wavelength of 228.8 nm in flame mode using air-acetylene flame. Standard and blank samples were run after every ten samples to check the AAS instrumental drift. The pH meter PHEP, Hanna Instrument from Italy was used to measure the pH in this study at a temperature of 25.0°C. Turbid Meter (2100P HACH) was used to determine the turbidity of water. Fourier transform infra-red machine (Perkin Elmer 100, Waltham Ma; USA) was used to deduce the main functional groups of dry cilantro leaves and stems. Calibration curve method was used to quantify the heavy metal ions concentration.Statistical Testing: The data from the study was treated by Analysis of variance (ANOVA) and Student Newman-Keuls (SNK) test for significance. Mean values that are followed by the same superscript letter (s) i.e. a, b, c and d within the same row do not differ significantly at 95% confidence level otherwise they differ significantly as SNK-test in tables for the percentage removal in appendices 2 (Kothari, 2004).Batch Experiments: The batch parameters under the study were initial metal ions concentration, dosage, pH, shaking speed, temperature and contact time on adsorption. The quantity of Cd2+ ions adsorbed per unit mass of the adsorbents and their percentage removed were evaluated using the equations 3 and 4 respectively;
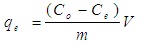 | (3) |
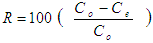 | (4) |
3. Results and Discussion
- Functional Group Analysis of Cilantro: Fourier transform infra-red (FTIR) spectroscopy was used to deduce the main functional groups in cilantro biomass of dry leaves (DL) and dry stems (DS). The IR spectrum of cilantro biomass is given in figure 1.
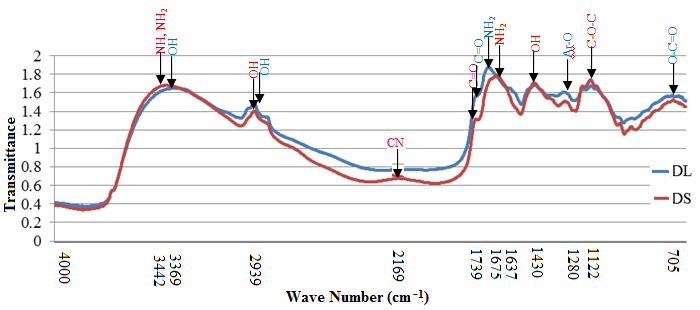 | Figure 1. FTIR spectrum of Dry Leaves (DL) and Dry Stems (DS) |
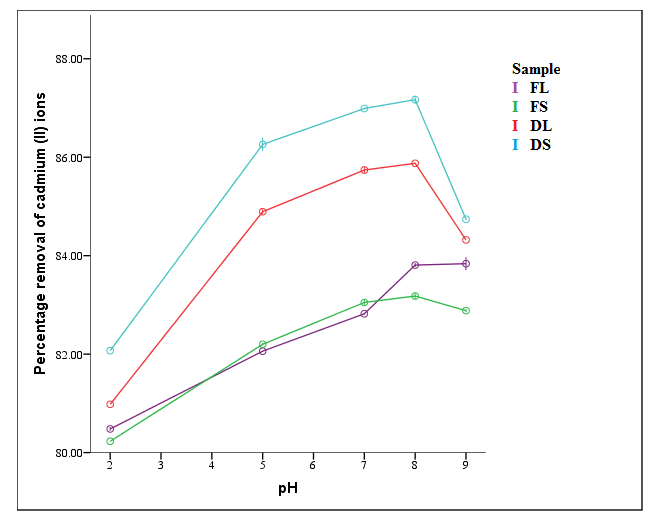 | Figure 2. Effect of pH on percentage removal of Cd2+ (T = 298 K, time = 2 hrs, concentration = 100 mg/L, dosage = 0.2 g, shaking speed = 6 rps) |
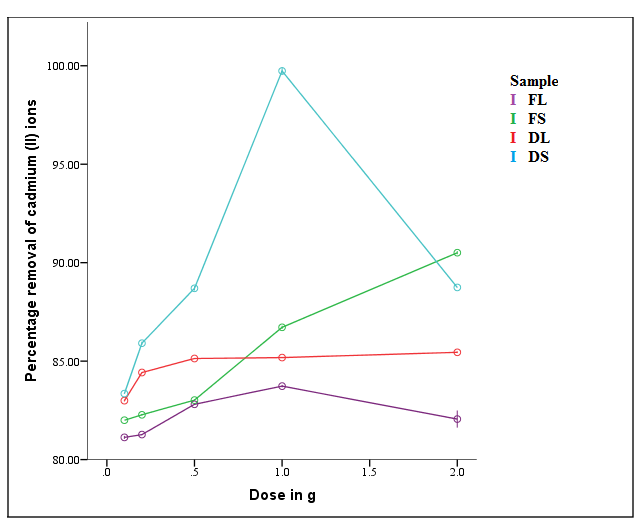 | Figure 3. Effect of adsorbent dosage on percentage removal of Cd2+ ions (T = 298 K, time = 2 hrs, concentration = 100 mg/L, pH = 6.0, shaking speed = 6 rps) |
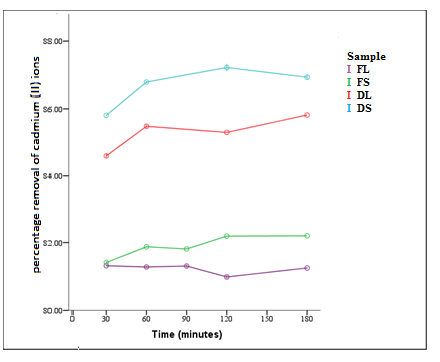 | Figure 4. Effect of contact time on percentage removal of Cd2+ (T = 298 K, pH = 6, concentration 100 mg/L, dosage = 0.2 g, shaking speed = 6 rps) |
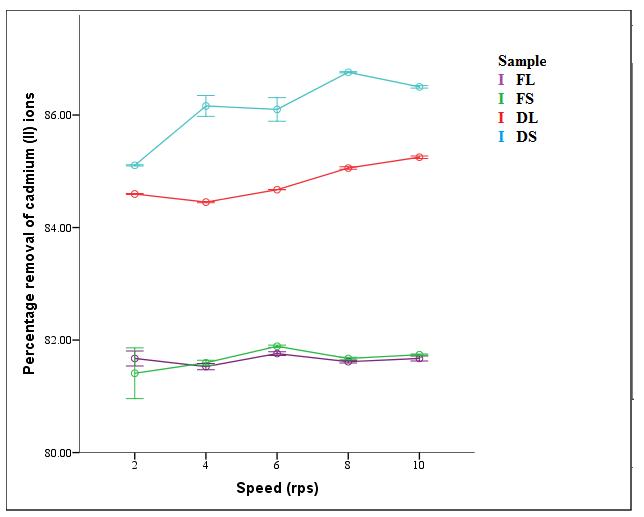 | Figure 5. Effect of shaking speed on percentage removal of Cd2+ (T = 298 K, time = 2 hrs, concentration = 100 mg/L, dosage = 0.2 g, pH = 6) |
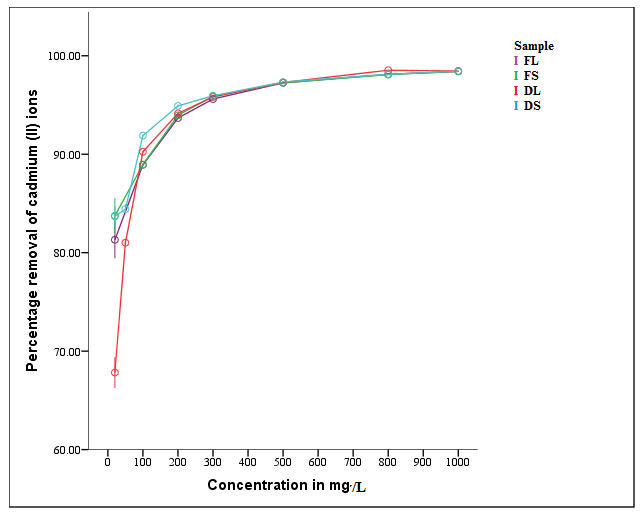 | Figure 6. Effect of initial metal concentration on percentage removal of Cd2+ (T = 298 K, time = 2 hrs, shaking speed = 6 rps, dosage = 0.2 g, pH = 6) |
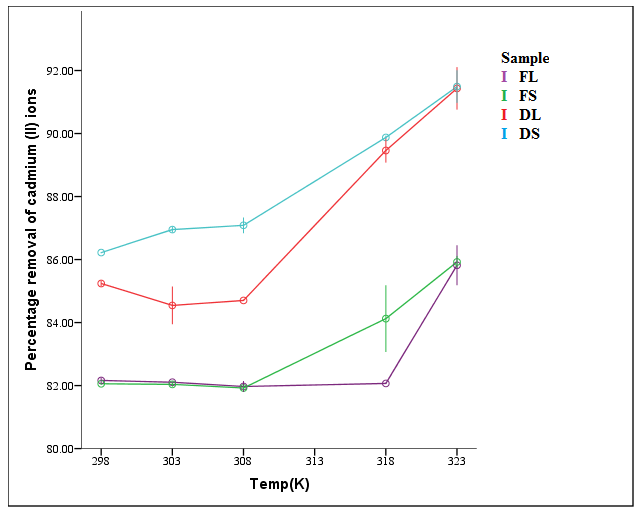 | Figure 7. Effect of temperature on percentage removal of Cd2+ ions (time = 2 hrs, shaking speed = 6 rps, dosage = 0.2 g, pH = 6, concentration = 100 mg/L) |
|
4. Conclusions
- The adsorption of Cd2+ ions was best explained by Langmuir adsorption isotherm with Qmax values of 250.00, 51.28, 4.68 and 15.06 mg/g for FL, FS, DL and DS respectively. The percentage removal efficiency values for FL and FS for Cd2+ ions for all the parameters under batch studies ought to been much higher, since 0.2 g of FL on drying gave 0.017 g dry biomass with a moisture content of 91.5%, while 0.2 g of DS on drying gave 0.1 g of dry biomass with moisture content of 50%; considering that all adsorbents dosage was maintained at 0.2 g during the batch adsorption studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML