-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2019; 9(1): 13-18
doi:10.5923/j.env.20190901.02

An Investigation on the Effects of Copper on Chenopodium album
Andile Tonga1, Jennifer Laifa2, Nomagugu Gxaba1, Gloria Miller3, Maria Begonia3, Gregorio Begonia3
1Department of Biological & Environmental Sciences, Walter Sisulu University, Nelson Mandela Campus, Mthatha, South Africa
2Department of Natural Sciences & Environmental Health, Mississippi Valley State University, Itta Bena, Mississippi, USA
3Plant Physiology/Microbiology Laboratory, Department of Biology, College of Science, Engineering, and Technology, Jackson State University, Jackson, Mississippi, USA
Correspondence to: Jennifer Laifa, Department of Natural Sciences & Environmental Health, Mississippi Valley State University, Itta Bena, Mississippi, USA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Copper (Cu) is an essential metal that is needed by humans, animals and plants. The metal has been found to be an irritant to humans. The gastrointestinal effects of copper in humans include nausea, anorexia and diarrhea. Excess copper in plants cause chlorosis, stunted growth, and denaturing of macromolecules. Wild plants are crucial in providing food source for humans. But at times, the wild plants can grow in contaminated soils. Chenopodium album from Chenopodiaceae is a wild leafy vegetable that is consumed in some parts of Eastern Cape, South Africa. It is believed to be rich in proteins, carotenoids and vitamin c. The hypothesis of the study was that Chenopodium album, a leafy wild vegetable consumed by humans in the Eastern Cape, South Africa would uptake and accumulate copper in the harvestable parts. The shoot heights, root lengths, shoot and root biomass and the concentration of copper were measured from C. album growing from soils contaminated with different concentrations of copper. Results revealed that the harvestable parts of C. album have accumulated 160.074 mg per kilogram copper. The results indicate that humans that consume C. album that is growing on copper-contaminated soils are at risk of being negatively affected by copper.
Keywords: Copper, Chenopodium album, Wild leafy vegetables
Cite this paper: Andile Tonga, Jennifer Laifa, Nomagugu Gxaba, Gloria Miller, Maria Begonia, Gregorio Begonia, An Investigation on the Effects of Copper on Chenopodium album, World Environment, Vol. 9 No. 1, 2019, pp. 13-18. doi: 10.5923/j.env.20190901.02.
1. Introduction
- Traditional vegetables are known to be plants that are consumed as vegetables because of their nutritional leafy parts, which include young succulent stems, leaves and their young fruits [1]. Consumption of traditional vegetables is essential because of their nutritional richness and minerals which are useful in maintaining human health [2]. In South Africa more than one hundred different species of plants are consumed as leafy vegetables [1]. These traditional vegetables are the cheapest sources of nutrition that are available to the people mostly in the rural areas of South Africa and also in some African and Asian countries.Although traditional vegetables have these benefits, they can grow in soils that are disturbed including those that are contaminated by heavy metals. An example of a traditional vegetable is Chenopodium album known as a pigweed, imbikicane, and imifuno [3-5]. Chenopodium album is an annual plant belonging to the Chenopodiaceae [3]. In the rural areas of Eastern Cape in South Africa, it is consumed as traditional vegetable and also used for livestock feed, because the C. album leaves are nutritionally rich and contain antioxidants and other important dietary elements [6]. C. album is one of the plant species that can grow in heavy metal-contaminated soils. This poses a great health concern of heavy metal poisoning since the plant is consumed by people as a source of nutrition [7]. Copper is one of the essential elements that are required in small amounts for the activation of enzymes during the synthesis of lignin. However, the ability of C. album to colonize copper-contaminated soils is a major health concern as copper causes damage to the liver [8].C. album is a wild vegetable that can grow in copper-contaminated soils, hence it poses a potential health risk as it is largely consumed by people as a leafy vegetable. There are few studies that have been conducted about C. album and its ability to accumulate copper [9]. This study is important by alerting people about the health risks they are exposed to when consuming the plant growing in copper-contaminated soils. Moreover this provides more information about the plant being used as an accumulator of copper which will help in minimizing copper contamination from the soil. The study was done to close the gap on health risks that result from consumption of C. album growing in copper-contaminated soils. There was a study that was conducted in 2015 at the Ntabankulu area where C. album was found to be one of the wild vegetables consumed by people. The study investigated whether humans are at risk of consuming C. album when found to accumulate copper since copper is one of the heavy metals found from that area.The aim of the study was to investigate the uptake and translocation of copper by C. album. We hypothesized that C. album accumulates copper and translocates it into different organs. To reach the set aim of the study, three objectives were to (a) determine the effects of copper on the growth and development of C. album, (b) identify the parts of the plant that can accumulate copper, and (c) analyze the concentration of copper in C. album.
2. Materials and Methods
- The study was conducted in the Department of Biological and Environmental Sciences at Walter Sisulu University, Nelson Mandela Drive Campus, Mthatha Eastern Cape South Africa.
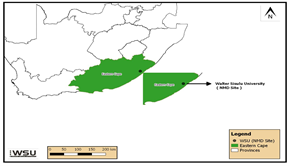 | Figure 1. Study area |
3. Results
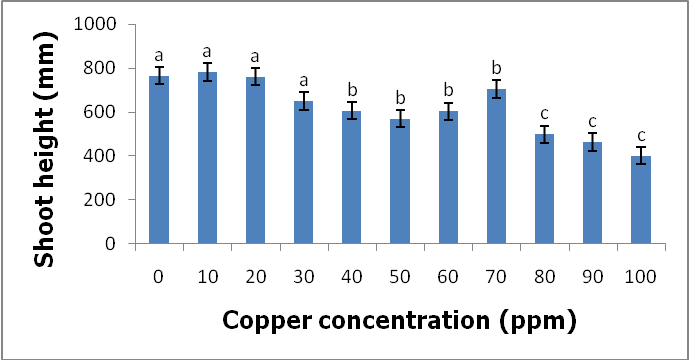
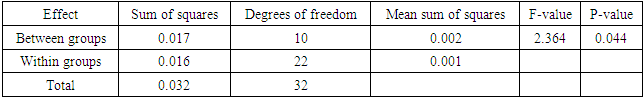
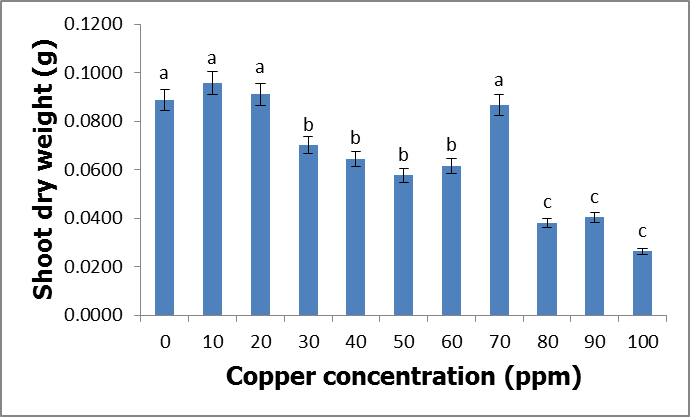
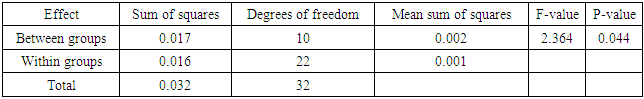
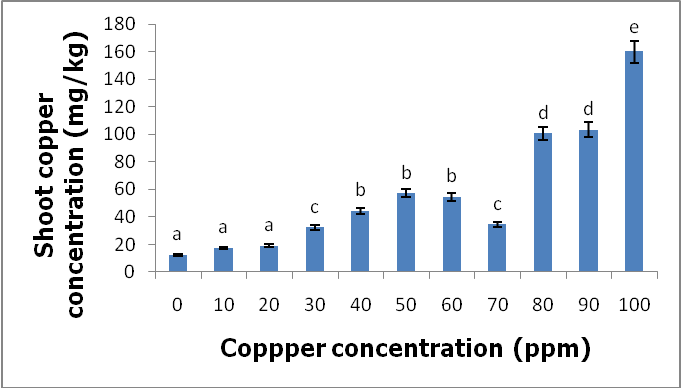
- Effects of copper on growth and developmentC. album grown in copper-contaminated soils showed no symptoms of copper contamination. However, there were few plants that showed signs of copper toxicity such as stunted growth and the yellowing of the leaves. Although the plants were exposed to different copper concentrations for a period of nine weeks, they continued to grow and developed well.Shoot heightsThe results obtained by comparing the shoot heights of C. album (Figure 2, Table 1) showed that there were significant differences between the shoot heights of C. album grown in copper-contaminated soils because p<0.05 at 0.004.
|
|
|
|
|
|
4. Discussion
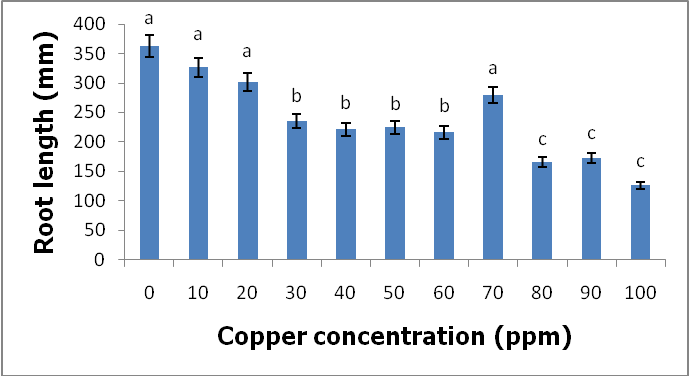
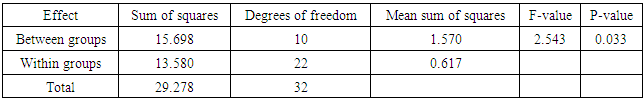
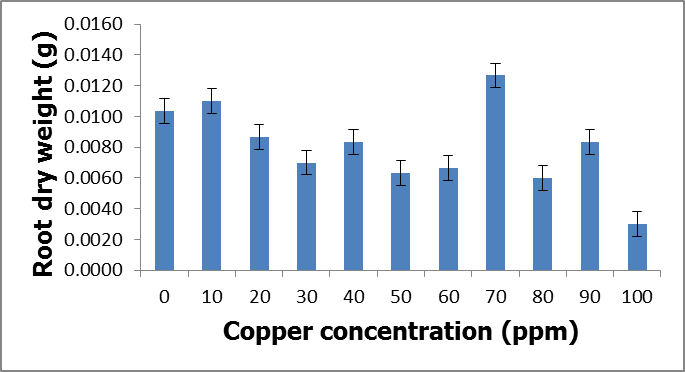
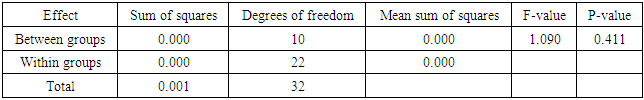
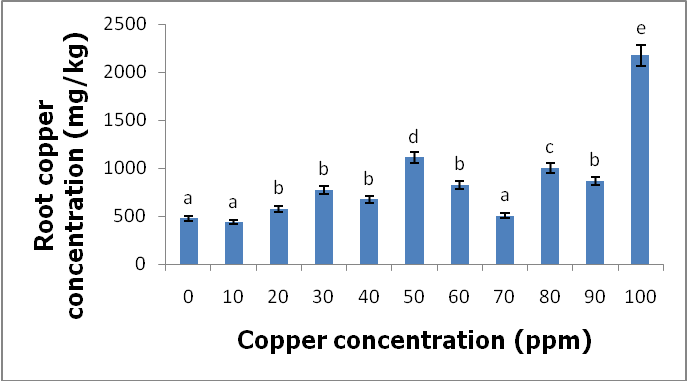
- Heavy metals occur naturally in the soil in different concentrations. These heavy metals are important in the soil as they play important roles in the plants’ growth and development when available at minimum levels in the soils. The release of these heavy metals in the environment due to human activities such as industrial waste, bactericides and fungicides lead to the increase of heavy metal concentrations in the soil. Their increase has a negative impact on the soil, agriculture and human health. Copper is an important micronutrient that is required by plants in small amount for their growth and development. When copper is available in excess amounts in the soil, it induces stress and causes damage in plants. Symptoms of excess copper in the plants include growth retardation due to the inhibition of roots elongation and leaf chlorosis [10].When plants are exposed to heavy metal-contaminated soils they tend to accumulate the elements from the soil regardless of the concentrations that are available in the soil. When the heavy metals are available at higher concentrations in the soil, they affect the fertility of the soil reducing the crop yields. Wild vegetables on the other hand are consumed as an alternative source of nutrition in the rural areas of South Africa and other African countries. In Europe some of the traditional vegetables are cultivated and used to produce flour [3].Wild vegetables grow in disturbed environments to some extent that they can grow in heavy metal-contaminated soils. This ability of thriving in heavy metal-contaminated soils raises health concerns, because plants that are exposed to heavy metal-contaminated soils tend to accumulate the elements from the soil [11]. Consumption of food contaminated with heavy metals such as copper poses serious health risks such as liver damage [8].In the present study, copper had no negative effects on the growth and development of the plants since there were no symptoms of copper toxicity such as stunted growth and leaf chlorosis. The present study is in contrast with the study by [12] which showed that copper had visible symptoms of morphological alterations such as the shortening of the stem and the chlorotic spots on the leaves.In this study the different copper concentrations were inversely proportional with the shoot heights. As the copper concentration increased from 10 ppm up to 100 ppm, the shoot heights of the C. album started to slightly decrease to the point whereby the shortest shoot height was recorded at 100 ppm. This study is in contrast with the study by [13] where the different copper concentrations had no effects on the shoots heights of the plants. From the same study by [13] the shoots of plants grown in different copper concentrations were more or less the same as the control plants. Similar outcomes were also recorded in the root lengths even though there were visible symptoms of copper toxicity such as the black tips and yellow appearance.In the present study, the shoot phytomass of C. album decreased accordingly with increasing copper concentrations in the soil. This study is in agreement with that of [14] who pointed out that shoot phytomass decreased with increasing concentration of copper in the soil, even though minimal amounts of copper were translocated from the roots to the shoots. Copper had no effect on the root phytomass of C. album. This study is in contrast with the study by [15] which showed that root phytomass of plants grown in different copper concentrations decreased with increasing copper concentrations due to the inhibition of root elongation and growth of root hairs by copper toxicity.In the present study copper accumulation in the roots and the shoots significantly increased with the increasing copper concentrations in the soil. These results are in agreement with the study by [11] which showed that the accumulation of copper in the roots and shoots increased with the increasing copper concentrations in the soil. The results for this study showed that the roots of the C. album accumulated more copper than the shoots.Wild vegetables are a good source of nutrition and can grow in heavy metal-contaminated soils. This is in agreement with the present study where C. album was grown in copper-contaminated soils and developed well without any visible symptoms of copper toxicity. The study revealed that C. album can accumulate copper. The accumulation of copper by C. album increased with increasing copper concentrations in the soil. The study also revealed that after root uptake, copper was translocated to the shoots.
5. Conclusions
- C. album growing in copper-contaminated soils for nine weeks showed no visible symptoms of copper toxicity. However, the shoot heights and root lengths decreased with increasing copper concentrations. Copper had an effect on the shoot phytomass of C. album. More specifically, as the copper concentrations increased, the shoot phytomass slightly decreased and the lowest mass recorded at 100 ppm. However, root weight was not affected by different copper concentrations. Accumulation of copper by C. album increased with increasing copper concentrations in the soil. The amount of copper accumulated in the roots was higher than in the shoots. The highest amount accumulated in the roots was 2175.833 mg/kg and the highest amount accumulated in the shoots was 160.074 mg/kg. The accumulated copper by plants was higher than the accepted amount of copper accumulation by plants and vegetables which is 10 mg/kg of copper. Copper is an essential element that is crucial to both plants and animals when available in small amounts. Consumption of wild vegetables growing in copper-contaminated soils may cause serious health problems. Soil contamination due to heavy metals is a major problem in agriculture and it is a major concern for public health because when these heavy metals are available in toxic levels they affect the growth and development of the plants. They also cause serious health problems when consumed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML