-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2017; 7(2): 49-56
doi:10.5923/j.env.20170702.03

Heavy Metal Concentration in Vegetables Grown around Dumpsites in Nairobi City County, Kenya
Joan M. Njagi1, Daniel N. Akunga1, Martin M. Njagi2, Mathew P. Ngugi2, Eliud M. N. Njagi2
1Department of Environmental and Occupational Health, Kenyatta University, Nairobi, Kenya
2Department of Biochemistry and Biotechnology, Kenyatta University, Nairobi, Kenya
Correspondence to: Joan M. Njagi, Department of Environmental and Occupational Health, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Solid wastes management constitute a major challenge for human and environmental health especially in developing economies. Use of abandoned open solid waste dumpsites for cultivation of vegetables and edible fruits has been witnessed in urban settings without consideration for the health hazards this may cause. This has been the case in Kenyan urban settlements; where abandoned dumpsites are used as sources of nutrient rich soils for cultivating mainly vegetables and other edible crops. How this phenomenon affects the heavy metal concentration in the edible parts of these plants has not been extensively researched and documented. Research has shown that vegetables grown in dumpsites are capable of accumulating high levels of heavy metals from contaminated and polluted soils with deleterious health effects for the consumers of these vegetables through the disruption of numerous biochemical processes. The aim of this study was to determine heavy metals concentration in the different types of vegetables grown around the abandoned Kadhodeki dumpsite in the outskirts of Nairobi city. Equal samples of edible vegetables were carefully harvested from three sites; upstream, midstream and downstream, and prepared for laboratory analysis. Heavy metal determination was carried out using Energy Dispersive X-ray Fluorescence (EDXRF) analytical technique as described in International Atomic Energy Agency document for analysis of geological materials. The results indicate that the vegetables under study had low levels of essential metals Fe, Mn, Zn and Cu while they had higher levels of Ni, Co, V, and more than maximum allowable levels (MAL) of Hg and Pb. This implies that consumption of vegetables from around the dumpsites may possess serious health challenges to the consumers.
Keywords: Dumpsites, Vegetables, Heavy metals, Contamination
Cite this paper: Joan M. Njagi, Daniel N. Akunga, Martin M. Njagi, Mathew P. Ngugi, Eliud M. N. Njagi, Heavy Metal Concentration in Vegetables Grown around Dumpsites in Nairobi City County, Kenya, World Environment, Vol. 7 No. 2, 2017, pp. 49-56. doi: 10.5923/j.env.20170702.03.
Article Outline
1. Introduction
- Nairobi City County faces many challenges in solid waste management as evidenced by uncollected garbage littering many of the city’s settlement sites which eventually find its way into open dumpsites. These unsightly areas pose a risk to the environment and public health mainly due to chemical, physical and biological contaminants present in the waste. Chemical contamination especially of heavy metals is of particular concern due to the chronic nature of the health impacts associated with their consumption. The problem of solid waste management in Nairobi City County may be attributed to a large extent to the influx of populations into the city without matched planning for anticipated increase in solid waste generation [1, 2].Though large amounts of the solid waste comprises of organic material, considerable proportions of plastic, paper, metal rubbish and batteries that are known to be real sources of heavy metal do exist [3-5].Heavy metals are defined as those with a high density of more than 5 mg mL-1. However the collective term now includes arsenic, cadmium, chromium, copper, lead, nickel, molybdenum, vanadium, iron and zinc [6]. Some metals also known as micronutrients, which include zinc, copper, nickel, chromium and iron have important physiological functions in all forms of life and are therefore essential for their survival [6, 7]. Research has shown that the soil in and around the dumpsite is usually nutrient rich as well as heavily contaminated with toxic heavy metals [8]. These heavy metals have been known to leach from solid wastes improperly disposed off in dumpsites causing environmental and health hazards due to their toxicity and their persistence nature [9]. Studies have also reported increased heavy metal concentration in dumpsite soils as compared to normal agricultural control sites [7, 8, 10]The nutrient content of wastes found in the dumpsites makes them attractive as fertilizers, but when untreated waste is used in crop production, it places consumer’s health at risk [11]. Trace metals may enter the human body through consumption of contaminated drinking water or ingestion of soil or crops grown on contaminated land [12, 13]. Heavy metals are non-biodegradable, persistent and can accumulate in soils even at low levels to toxic concentrations. According to [14] these metals are then bio-available along the food chain thus affecting plant and animal life resulting in long-term cumulative health effects which are among the leading health concerns all over the world [15-17].World Health Organization (WHO) estimates that about a quarter of the diseases facing mankind today occur due to prolonged exposure to environmental pollution [18]. According to [2], the burden of disease associated with environmental heavy metal contamination has seen a dramatic increase in the past three decades. Most of these environment-related diseases are however not easily detected and may be acquired during childhood and manifested later in adulthood [19]. Metals such as lead, mercury, and cadmium are cumulative poisons that cause environmental hazards and are reported to be exceptionally toxic even in very small quantities [20]. They are a major source of oxidative stress in the cell and play an important role in the aetiology of diverse human pathologies such as carcinogenesis [21-24]. The general belief that waste is sometimes hazardous to health cannot therefore be overemphasized.The management of solid waste in Kenya and Nairobi city at large has been largely through indiscriminate open dumps. Kadhodeki dumpsite located in Waithaka Sub location is one of such sites. The dumping started in 1986 as a way of filling in the large gaping man holes that had been left open after quarrying activities in the construction of Nairobi/ Waiyaki highway. All types of solid waste continued to be indiscriminately dumped in the area till its unofficial closure in 2007. The abandoned dumpsite has since seen a dramatic increase in the variety and intensity of farming activities carried out by farmers around the sub location as a means of earning a living.This study therefore attempted to assess the levels of heavy metals in vegetable crops grown on and around the dumpsite. Information generated will be of great benefit to communities around the dumpsite, public health and environmental health policy makers.
2. Materials and Methods
2.1. Study Location
- Nairobi City County hosts the capital city of Kenya. It lies at an altitude of 1,670 meters above sea level and occupies an area of 696 km2. It is divided into 17 administrative sub-counties. This study was carried out in Kadhodeki Village in, Waithaka Sub location, Dagoretti South Sub County Nairobi City County, Kenya. The village is home to Kadhodeki Dumpsite, an open dumpsite declared illegal by the Nairobi City Council. The coordinates for the study area are 1016'31.58"S, 36043’52.95"E (the red patch shows the area occupied by the dumpsite; all units are in square metres).
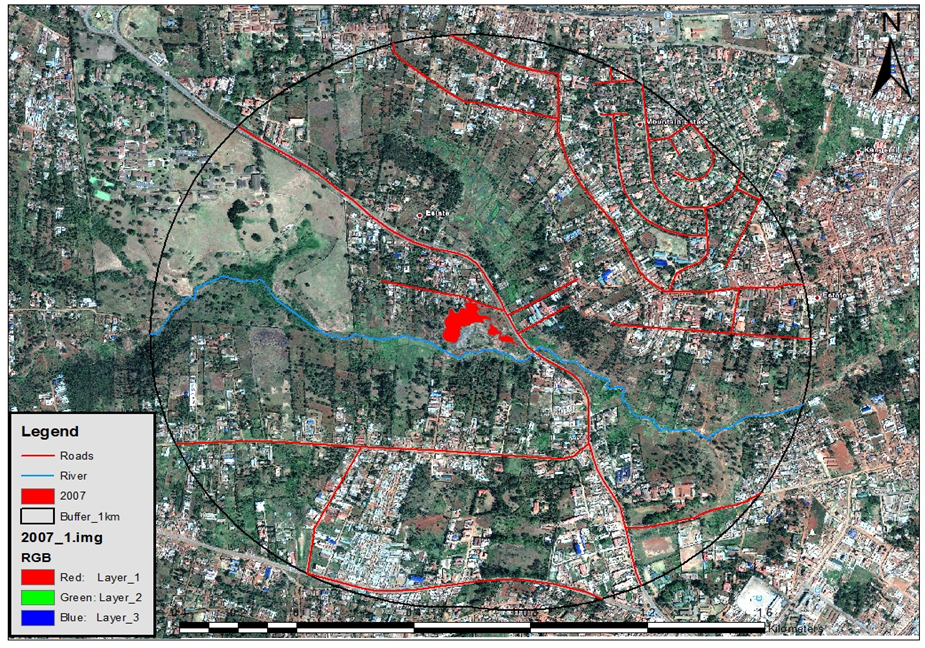 | Figure 1. Map of the study site showing the dumpsite (red) patch and the Nairobi River (Blue line) |
2.2. Plant Sample Collection and Preparation
- The study focused on edible leafy vegetables grown in and around the dumpsite mainly for commercial purposes. The edible plants of interest were Spinacia oleracea, Brassica oleracea Acephala and Solanum Villosum. These three are green leafy vegetables that grow in many areas in the country and form a part of everyday meals to the majority of Kenyans of all walks of life. They mature very fast and are affordable to all. The study area was divided into three sampling areas (upstream, midstream and downstream). These divisions were necessary to observe the effect of nature, pollution and dilution effect over time. Upstream was relatively natural, midstream the dumping area and downstream a lower gradient to the dumpsite. Two right angle intersecting transect lines were drawn in each of the sampling sites (upstream, midstream and downstream). Four sample batches of the leafy edible vegetables growing along the transect lines were carefully picked during the months of January and February 2011. The upstream and downstream sampling sites were 300 metres equidistant from the midstream (dumpsite) and running parallel to Nairobi River fig 1).The following vegetables were collected/ harvested at maturity; Spinacia oleracea, Brassica oleracea Acephala and Solanum Villosum. Immediately after harvesting and while still in the field, the vegetables were placed under running tap water to wash off soil particles and other debris then rinsed off with distilled water. After initial shade drying the samples were then packed into clean polythene bags for laboratory preparation. At the laboratory they were then cut into small pieces and then air dried at room temperature in enclosed chambers for about two weeks and then pulverized to fine powder using a stainless grinder. Ground plant samples were then collected in labelled polythene bags and were placed in a desiccator awaiting laboratory analysis [25, 26].
2.3. Determination of Heavy Metal Concentration in Plant Samples
- This was done using Energy Dispersive X- Ray Fluorescence Spectroscopy (EDXRF) technique. Ten grams of vegetable and 5 grams of starch were mixed and pressed into 7 mm disc in thickness and 4.1 cm in diameter in a mould with a force of 200kN for 5 sec. In order to retain the original dry mass of the samples, the discs were stored in desiccators till EDXRF analyses were done. The above procedure was repeated four times for each vegetable. The mineral element concentrations were determined by EDXRF analyses using ray EDX-720, EDXRF spectrometer. The following elements were quantified; V, Mn, Fe, Co, Ni, Cu, Zn, Hg and Pb. The results were expressed as milligrams per kilogram (mg/kg) of the dry matter.The heavy metal data was analysed using Microsoft excel to get the mean and standard error of the mean which was then subjected to statistical tests of significance using ANOVA (p<0.05). The results that were found to be statistically significant were subjected to a Tukeys post hoc analysis test (XL Daniel’s toolbox version 4.01 for post ANOVA). Mean heavy metal results were then extrapolated to calculate the estimate for calculated daily intake of metal (DIMetal) [27].
3. Results
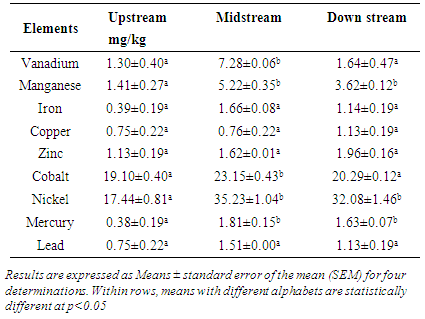
3.1. Mean Heavy Metal Concentrations in Spinacia oleracea
- According to the results shown on table 1 below, vanadium and cobalt metal concentration in Spinacia oleracea increased from the levels indicated upstream to highest levels in vegetables that were grown next to the dumpsite. The content however decreased in the vegetables grown downstream, to a similar level as that displayed by the vegetables grown at the control site. Manganese, nickel and mercury showed a different pattern whereby the content of these three metals in the Spinacia oleracea collected at the upstream was low. The levels increased to maximum in the vegetables grown next to the dumpsite. The heavy metal content remained at a constant as indicated by the concentrations of the metals in the Spinacia oleracea grown downstream. Iron, copper, zinc and lead mean levels in the Spinacia oleracea did not statistically change from the levels shown for vegetables grown upstream to those grown next to the dumpsites and those grown downstream. The results show an increase in the heavy metals for the vegetables grown next to the dumpsite.
|
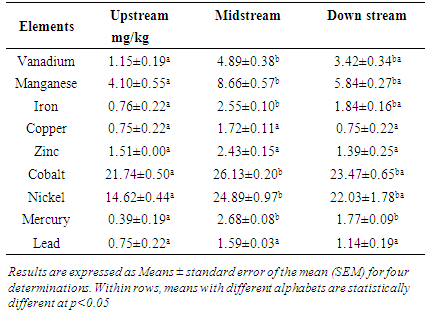
3.2. Mean Heavy metal concentrations in Solanum villosum
- Table 2 below shows that the pattern of heavy metal concentration displayed by vanadium, manganese, iron, cobalt nickel and mercury for Solanum villosum at the three sites is similar. The content of these metals in the Solanum villosum collected upstream was low. The levels increased to maximum in the vegetables grown next to the dumpsite, then remained at a constant as indicated by the concentrations of the metals in the vegetables grown downstream. Copper, zinc and lead heavy metal levels in the Solanum villosum did not change across the three sites. There was a general increase in the heavy metal content for the vegetables grown next to the dumpsite.
|
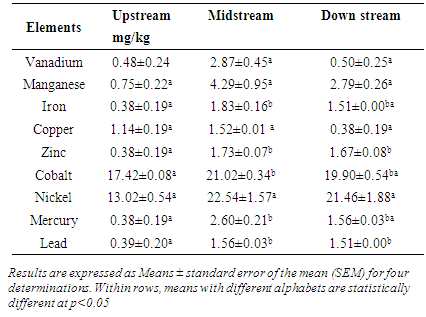
3.3. Mean Heavy Metal Concentrations in Brassica oleracea Acephala
- The table 3 below shows that the pattern of heavy metal concentration displayed by iron, cobalt, zinc, lead and mercury for Brassica oleracea Acephala at three sites is similar. The content of these metals in the vegetables collected upstream was low. The levels increased to maximum in the vegetables grown next to the dumpsite, then remained at a constant as indicated by the concentrations of the metals in the vegetables grown downstream. Vanadium, manganese, copper and nickel heavy metal concentrations in the Brassica oleracea Acephala did not change across the three sites. Although there was no statistical significance in heavy metal concentration, the figures show an increase in the levels from those vegetables collected upstream to those collected next to the dumpsite.
|
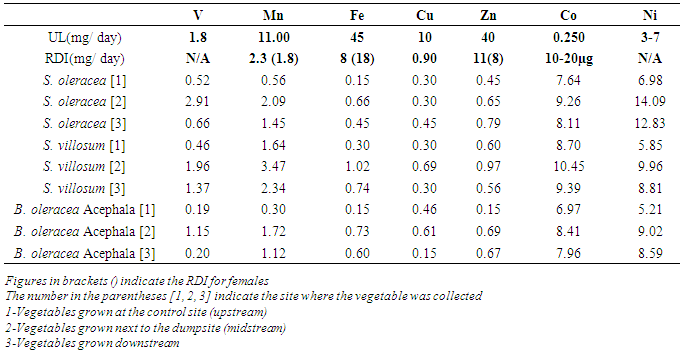
3.4. Extrapolation of Daily Metals Intakes Estimate (DImetal)
- The daily metals intake estimate (DIMetal) of Fe, Mn, Zn, Cu, Ni, Co and V from each vegetable in this study were calculated by multiplying the daily recommended vegetable intake (400g) by the metals concentrations of the vegetables. The DImetal were then compared with the recommended daily intakes and or allowances and the upper tolerable daily intakes for the metals (UL) [28-31]. Table 4 below shows that the DImetal values for zinc, copper and iron were below the recommended daily allowance of 11(8) mg/day, 0.9 mg/day and 8(18) mg/day, respectively. The vegetables grown in the three sites had DImetal vanadium values below the set upper limit of 1.8 mg/day except from Spinacia oleracea and Solanum villosum grown next to the dumpsite which recorded values higher than 1.8 mg/day.
|
4. Discussion
- In this study, the mean metal concentrations of manganese in the vegetables ranged from 0.75±0.22 to 8.66±0.57 mg/kg. This agrees with a study carried out by [32] who reported manganese values of between 1.38 to 10.47 mg/kg in the vegetables studied. However, [33] reported higher values of 5.23 ± 0.06 to 11.75 ± 1.04 mg/kg in the vegetables. The DIMetal values for manganese in the vegetables were found to be higher than the recommended daily intake, however, the values were below the tolerable upper intake level limits. Manganese is required as a catalytic cofactor for mitochondrial superoxide dismutase, arginase, and pyruvate carboxylase and it is also an activator of several other enzymes. Extremely high levels of manganese exposure have been shown to affect brain development and cause severe symptoms of manganism disease [34, 35].The mean metal concentration ranges of nickel in the vegetables studied reported similar findings as those of [36, 37]. On the other hand, [38] in Bangladesh reported lower levels of nickel than those of this study. Studies by [39] have shown that vegetables have more nickel than animal products. In this study only the vegetables grown at the control site were within the reported safe range of 3-7 mg/day daily intake.Intake of very large quantities of nickel by humans from plants grown on nickel rich soils has higher chances of inducing the development of cancers of the lung, nose, larynx and prostrate as well as inducing respiratory failures, birth defects and heart disorders [40].Studies have also shown that heavy metals such as nickel can stimulate cell growth in estrogen receptor (ER) positive breast cancer cells [41]. Indeed, [42] found highly significant nickel accumulation in 20 breast cancer tissue biopsies compared to controls. However, although nickel is a toxic metal, it plays a role as a coenzyme in different enzymes [29, 43]. The daily recommended range of cobalt in human diet is 0.005mg/day [44]. The mean metal concentrations of cobalt in the vegetables understudy ranged from 17.42±0.08 to 26.13±0.20 mg/kg. The DIMetal values recorded were above the recommended range, however, the values were below 30 mg/day which have been shown to cause digestive and skin disorders in humans [44]. Cobalt has been confirmed to be a carcinogen in animal studies and is considered a possible carcinogen in humans. Symptoms of cobalt toxicity include fatigue, nausea, vertigo and problems with balance, poor memory and cognitive function, tinnitus, blindness, headaches, cardiomyopathy, hypothyroidism, peripheral neuropathy with tremors and loss of coordination, rashes, kidney failure, anxiety and irritability [45].The mean metal concentration of vanadium in the three vegetables ranged from 0.48±0.24 to 7.28±0.06 mg/kg. [46] reported higher vanadium values than those of this study of between 31 to 35 mg/kg in plants. The vegetables were seen to have DIMetal values lower than the upper limit established by [30, 47-49].In this study, the mean metal concentrations of lead in the vegetables ranged from 0.39±0.20 to 1.59±0.03 mg/kg. [38, 50-51] reported lead levels in vegetables in ranges similar to those of this study. However, [52] reported higher lead metal levels in several vegetables. According to the maximum allowable limit for lead in vegetables of 0.5mg/kg as used by [32, 53], all the vegetables apart from Brassica oleracea Acephala grown at the control site were above this limit. The high levels of lead in the vegetables could be attributed to the dumpsite and the busy Uthiru / Kawangare Road. This is because in the past lead was used in gasoline and hence a major contributor to lead in soil, and eventually plants. The Kadhodeki dumpsite is located a few metres from the busy Nairobi/ waiyaki road (fig1). Indeed, a study carried out by [47] found high levels of lead in both soil and couch grass grown along the road in Dar es Salaam. Lead is released into the air during burning of oil, or waste and is removed from the air by rain and by particles falling to land or into surface water. Once lead falls onto soil, it sticks strongly to soil particles and remains in the upper layer of soil and can be accumulated by vegetables grown on such contaminated soils [48].Lead has no beneficial function and it is in fact known to accumulate in the body and cause adverse health effects. It is a neurotoxin that permanently interrupts normal brain development and accumulates in the skeleton and its mobilization from bones during pregnancy and lactation causes exposure to foetuses and breastfed infants [48]. On a cellular and molecular level, lead may enhance carcinogenic events involved in DNA damage, DNA repair and regulation of tumor suppressor and promoter genes [54].All the vegetables grown at the control site recorded mean concentration of less than 0.5 mg/kg of mercury. The highest level recorded for mercury was 2.68±0.08 mg/kg. Work done by [51, 55-56] reported values lower than those of this study.Mercury is a neurotoxin and long-term, low-level exposure to elemental mercury vapor, such as eating food contaminated with organic mercury, affects the nervous system most adversely, causing depression, anxiety, insomnia, constant fatigue, tremor, and behavioural disturbance [57].
5. Conclusions
- This study showed that the vegetables may pose a health risk to the people who consume them as they were found to be deficient of essential metals such as iron, zinc, copper and manganese. On the other hand, they were found to have higher than allowed levels of metals like cobalt, vanadium and nickel. Furthermore, the vegetables were also found to have high levels of toxic metals such as mercury and lead. The dumpsite was shown to directly contribute to the pollution of the vegetables and the fact that it is an illegal entity, as such dumping should be stopped and the site properly closed.
ACKNOWLEDGEMENTS
- The study would wish to acknowledge Mr. Kangethe a village elder from the study area and Mrs. Lydia from the Ministry of Agriculture Dagorreti division who accompanied us as we collected the laboratory samples. Kenya Bureau of Standards (KEBS) for the analysis of heavy metals in the vegetable samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML