-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2016; 6(1): 10-18
doi:10.5923/j.env.20160601.02

Metal Uptake, Growth Responses, and Chlorophyll Production of Wheat (Triticum aestivum) Exposed at Different Durations to Chelate-Amended Cadmium-Contaminated Soils
Gloria Miller, Charles Davis, Gregorio Begonia, Maria Begonia
Plant Physiology/Microbiology Laboratory, Department of Biology, College of Science, Engineering, and Technology, University, Mississippi, USA
Correspondence to: Gloria Miller, Plant Physiology/Microbiology Laboratory, Department of Biology, College of Science, Engineering, and Technology, University, Mississippi, USA.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Phytoextraction is gaining acceptance as a cost-effective and environmentally sound phytoremediation strategy for reducing toxic metal levels from contaminated soils. We hypothesized that the addition of synthetic chelates can increase the amount of bioavailable metal for root uptake, thereby improving the efficacy of phytoextraction. This study was conducted to evaluate the effects of Cadmium (Cd) and ethylenediaminetetraacetic (EDTA) on the growth, metal uptake, and chlorophyll (Chl) production in wheat plants. Wheat (Triticum aestivum L. cv TAM-109) seeds were planted in plastic tubes containing topsoil and peat (2:1, v: v) spiked with various levels (0, 250, 500 mg Cd/kg dry soil) of cadmium nitrate. After 30, 45, and 60 days of growth, plants were harvested. Results show that after 60 days, wheat biomass was greater at 100, 250, and 500 mg/kg of Cd-treated soil without the addition of EDTA as compared to plants grown in Cd-treated soil plus EDTA. Although Chl production generally decreased with increased exposure time and Cd concentration, we nonetheless observed Chl production to be higher during each harvesting period in plants exposed to Cd plus EDTA. Treatments with the highest Chl concentration occurred on day 30.
Keywords: Cadmium, Chlorophyll, Wheat, Phytoremediation
Cite this paper: Gloria Miller, Charles Davis, Gregorio Begonia, Maria Begonia, Metal Uptake, Growth Responses, and Chlorophyll Production of Wheat (Triticum aestivum) Exposed at Different Durations to Chelate-Amended Cadmium-Contaminated Soils, World Environment, Vol. 6 No. 1, 2016, pp. 10-18. doi: 10.5923/j.env.20160601.02.
Article Outline
1. Introduction
- Cadmium (Cd) is a non-essential heavy metal (HM) pollutant resulting from agricultural and mining activities as well as from industrial and commercial uses. Cadmium is highly toxic and has a high solubility potential [1]. The quality of soil, nutrient cycling, and agricultural production are affected by the presence of Cd [2-7]. Fortunately, there are several safeguards in place to help protect public health. For example, the Environmental Protection Agency (EPA) has set a limit of 5 parts of Cd per billion of drinking water and does not allow any Cd in pesticides. The Food and Drug Administration (FDA) limits the amount of Cd in food colors to 15 parts per million (ppm). The Occupational Safety and Health Administration (OSHA) limits workplace air to 100 µg/m3 as Cd fumes, and 200 µg /m3 as Cd dust. It has also been determined by the Department of Health and Human Services (DHHS) that Cd and Cd compounds may reasonably be anticipated to be carcinogens. While it is not known whether Cd exposure can affect development in humans, it has, however, been found in animal studies, that the offspring of mice exposed to Cd during pregnancy had delayed development. Also, Cd causes kidney damage of mammals, as well as emphysema and acute lung condition [8]. From the mammalian toxicity data of elements in injected doses and diets, 1.3 mg/kg Cd has been determined to be the acute lethal dose (LD50). In human diets, 3 to 330 mg/day is the toxic dose [9]. Cognizant of the threats that HMs pose to the environment including human and animal health, there has been an increasing interest in phytoremediation as a plant-based alternative for cost-effective and environmentally sound clean up of heavy metal-contaminated soils [10-15]. The plant-based alternatives used in the management of metal-contaminated sites are those with adaptive biological mechanism (avoidance/exclusion and tolerance/ accumulation) that will allow them to resist or tolerate high concentrations of metals [16, 17]. The success of phytoextraction, a branch of phytoremediaton, depends upon the ability of plant species to absorb a substantial amount of HMs into their roots and preferentially translocate the metal into the harvestable above-ground biomass for easier harvesting. Monocotyledonous plants are thought to be excellent candidates because their fibrous root systems can stabilize soil and provide a large surface area for root-soil contact [18, 19]. It has been reported that plants that demonstrate growth inhibition have also exhibited low Chl concentrations / content in their leaf tissues. Since there is a correlation between Chl content and leaf area photosynthetic rate [20, 21], it is a popular assumption that reduction in Chl production can cause an overall inhibition in plant growth due to inadequate photosynthesis. Understanding this mechanism is important in phytoremediation since the rate of bioremediation is directly proportional to plant growth and total biomass. The present experiments were therefore designed to quantify the effects of Cd on Chl content, plant biomass, and Cd uptake. Chelates such as EDTA can bring more ions into solution, and that properly selected plants can provide enhanced surface area for absorption of HM ions and their chelated forms [22]. Radish, for example, has been reported to tolerate Pb concentrations as high as 240 ppm and accumulates significant levels of the metal in its shoots especially in conjunction with EDTA [23]. The main objectives of this study were, therefore, to further evaluate the effectiveness of chelates such as EDTA in bringing Cd ions into solution for phytoextraction and to further assess Chl production in Triticum aestivum L. cv. TAM-109 under toxic metal conditions.
2. Materials and Methods
- Cadmium nitrate [(Cd(NO3)2], and other chemicals were purchased from Sigma Chemical (St. Louis, MO), and Fisher Scientific (Houston, TX). Delta top soil and humus peat were purchased from Hutto's Garden Supply (Jackson, MS). Wheat seeds were obtained from a local garden store in Jackson, MS. Deepot D40 656 mL planting tubes (referred to hereafter as planting tubes or tubes) and support trays were purchased from Stuewe and Sons, Inc. (Corvallis, OR). Delta top soil and peat were weighed on a Brainweight 1500 scale, Model B1500 (Ohaus Scale corp.). Dry tissue biomass was determined using a Metler AE260 analytical balance. Reagents were weighed with a Wards Electronic top loading balance (Model 15 W6201). Plant tissue samples were dried in a Precision Thelco Model 18 convection oven at 75°C for approximately 48 hours. Cadmium concentration of plant tissues were analyzed with a Perkin Elmer Optima 3300 DV Inductively Coupled Plasma -Optical Emission Spectrometer (ICP-OES) and were expressed as µg Cd/g dry weight (ppm). Modified Hoagland's nutrient solution containing buffers and trace elements were prepared in the laboratory.
2.1. Growth Medium Preparation and Plant Establishment
- Delta top soil and humus peat were allowed to air dry to approximately 1 – 3% moisture content for 3 – 4 days under greenhouse conditions. Top soil and peat were cleaned of debris using a 1 cm sieve. Growth medium was prepared by mixing sieved soil and peat in a 2:1 volumetric proportion. Soil characterization: Representative soil samples of the prepared soil were sent to Mississippi State University Soil Testing Laboratory, Starkville, MS to determine parameters, such as soil texture, organic matter, pH, and nutrients in order to establish the soil characteristics and condition at the beginning of the experiment. Approximately 550 g of air-dried topsoil and peat mixture (2:1 v/v) were placed in a plastic zip lock bag and amended with either 0, 250, or 500 mg Cd/kg dry soil mixture using cadmium nitrate [Cd(NO3)2]. Deionized distilled water (DDW) was added to the bags to adjust the soil moisture content to approximately 30% field capacity. The bags of soil were left to equilibrate (age) on a laboratory bench in the greenhouse for six weeks. The bags were occasionally turned and mixed during the incubation period to ensure thorough mixing. The soil mixture, and aging process just described was used throughout the entire study. After the six-week equilibration process, the soil was placed in prepared 656 mL capacity D40 Deepot planting tubes. To prepare the planting tubes for the growth medium, a brown paper towel (Wipe-All C40) was folded and inserted into the bottom of each cell. The perforations were covered with scotch tape and parafilm to prevent rapid leakage and to allow aeration at the root zone.A 250 mL plastic cup was placed under each tube for leachate collection and to prevent cross contamination among treatments. Any leachate collected in each plastic cup was poured back into its corresponding tube. Periodically, these cups were rinsed with deionized distilled water (DDW) and the resulting washing solutions were poured back into the respective tube. The volume of water and/or nutrient solution ensured that soil moisture content was maintained at field capacity. An average of eight seeds were planted per tube. Emerged seedlings were thinned out to a desired population density of four plants per tube at 5 days after emergence. In this experiment, each treatment replicate consisted of one tube containing four plants. Any symptoms of metal toxicity (e.g., discoloration, pigmentation, yellowing, stunting) exhibited by plants were noted during the experimental period. Additionally, the position of each D40 tray was rotated one position clockwise each day or two to prevent any growth discrepancies that may occur due to shading by another plant. Plants were maintained at the naturally lit Jackson State University greenhouse throughout the experimental period. Each planting tube was watered with 10 mL of DDW as needed. After 15 days, the plants were watered with modified Hoagland’s nutrient solution on alternating days and maintained until harvested after 30, 45, or 60 days of growth. The chelating agent, EDTA, was applied as an aqueous solution one week before each harvest. After harvesting, the root and shoot lengths were measured, roots and shoots were separated and roots were washed with DDW to remove any adhering soil particle and debris. The roots and shoots were placed separately in brown paper bags and oven-dried at 75°C for 48 hours. The dry biomass of roots and shoots were determined using a Metler AE260 analytical balance.
2.2. Cadmium Extraction from Plant Tissues
- Cadmium was extracted from root and shoot samples using a nitric acid-hydrogen peroxide procedures with slight modifications. Briefly, 40 mL of 50% aqueous nitric acid were added to a representative 200 mg sample of plant tissue. The acidified sample was heated to ~ 35°C, refluxed for 15 minutes without boiling and then allowed to cool. Another 10 mL of 50% aqueous nitric acid were added and the sample was again heated and refluxed for 30 minutes. The heated sample was allowed to cool and then completely oxidized in 5 mL of concentrated nitric acid. The oxidized solution was then allowed to evaporate to approximately 5 mL without boiling. To initiate the peroxide reaction, 2 mL of DDW and 3 mL of 30% hydrogen peroxide (H2O2) were added to the concentrated digestate and then heated until effervescence subsided. Another 7 mL of 30% hydrogen peroxide were added continuously in 1 mL aliquots as the digestate was again heated. The digestate was heated until effervescence was minimal and its volume reduced to approximately 5 mL. After cooling, the final digestate was diluted to about 15 mL with DDW. The digestate was then filtered through a filter paper (Whatman No. 1) and the final volume was adjusted to 25 mL with DDW. Samples were analyzed for Cd concentrations with a Perkin Elmer Optima 3300 DV Inductively Coupled Plasma -Optical Emission Spectrometer (ICP-OES) and were expressed as µg Cd/g dry weight (ppm).
2.3. Chlorophyll Determination
- At harvest, chlorophyll (Chl) was extracted from sections of two leaves. The 1.0 cm leaf sections were taken from the inside, upper middle portion of two leaves of each wheat plant. Fresh weights were recorded and Chl extractions followed the methods of Knudson and colleagues [24]. This method involved immersing leaves in 30 mL 95% ethanol for 24 hr, then decanting the ethanol-Chl solutions to a second container. The leaves were soaked in a similar manner for two additional 24 hr periods with fresh aliquots of ethanol each time. Solutions were accumulated from each extraction and adjusted to a final volume of 100 mL. All Chl appeared to be extracted from the leaves, with most of the extraction occurring in the first of the three 24-hr periods. The ethanol-Chl solutions were kept in darkness at room temperature and immediately following the extraction sequence, absorbance (A) of each Chl extract was determined at 665 and 649 nm using a Milton Roy Spectronic 20 Spectrophotometer. The 1.0 cm leaf sections were then oven dried for at least 24 hr and dry weights were recorded and used to calculate µg Chl/mg dry weight. The ratio of Chl a to Chl b was also determined for each extraction, and all Chl values were rounded to two decimal places. Chlorophyll was quantified using the equation previously described by Knudson et al. [24], and others [25, 26]:µg Chl a = (13.70) (A 665 nm) - (5.76)(A 649 nm) mL solutionµg Chl b = (25.80) (A 649 nm) - (7.60)(A 665 nm) mL solutionTotal dry plant weights for Cd content were obtained by combining the dry weight of the 1.0 cm leaf sections and the dry weight of the leaves of the remainder of each plant sample. Plant tissue samples from Chl extractions were acid digested along with the shoot samples for Cd uptake calculations. All treatments were replicated 4 times.
2.4. Data Analyses
- In general, this study was arranged in a randomized complete block design (RCBD) and consisted of four replicates. Data were analyzed using SAS V9. Statistical analysis of variance was performed on all data sets. Least significant difference (Fisher's LSD) was used for multiple comparisons between different treatments and control groups. Results of the statistical data were summarized in figures and tables. In this study, a probability of 0.05 or less was considered to be statistically significant.
3. Results and Discussion
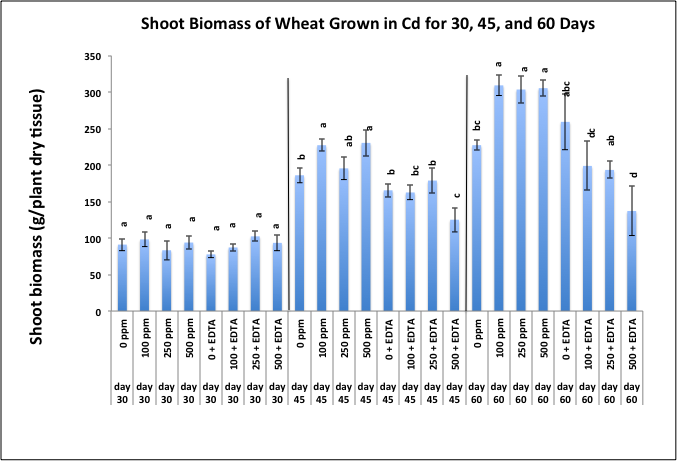
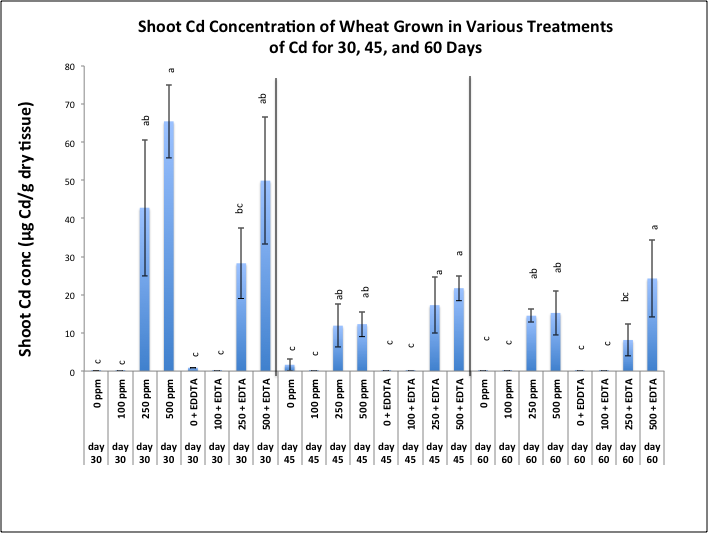
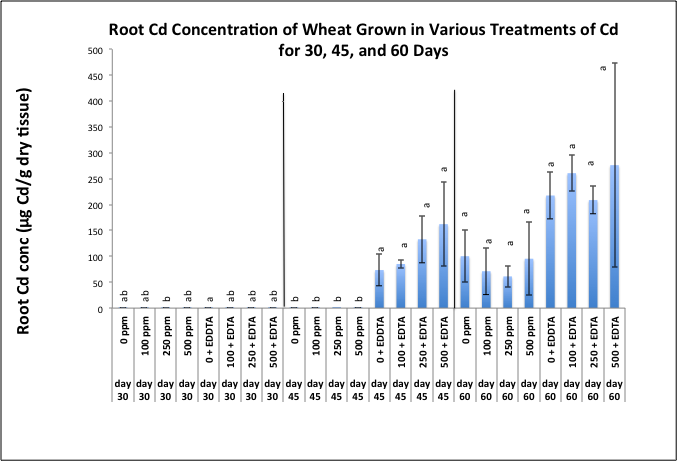
- Delta top soil used in this study was previously classified as a silt loam soil, belonging to the family of fine, Kaolinitic, thermic typic Kandiudult soils [27]. Representative samples of this soil mixture has been previously analyzed by the Mississippi State University Soil Testing Laboratory, Mississippi State, MS for determination of its physical and chemical characteristics (data not shown). The soil was determined to have a silt loam texture with a cation exchange capacity (CEC) of 17.6 and a pH of 6.3. The extractable magnesium was considered to be very high at 726 lbs/acre.In general, the chemistry of metal interaction with soil matrix is central to the phytoremediation concept. While the soil used in this study was high in phosphorus, potassium and zinc; and very high in magnesium, these were, nonetheless, essential plant nutrients. Overall, the parameters of the soil used in this study were well within limits for our objectives. The soil used in this study was classified as delta top soil, a silt loam soil which belongs to a family of fine, Kaolinitic, thermic typic Kandiudult soils. These soils have a low, but not extremely low cation-exchange capacity (CEC) [27]. Soil pH is one of the most influential parameters controlling the mobility and availability of metals in the soils. In our greenhouse study, soil pH before planting was 6.3 and decreased with increasing amounts of soil-applied Cd. After chelate application, the soil pH remained in the range of 5.5 - 6.5 (data not shown), a range not unusual in chelate-assisted phytoextraction [28]. Unlike nitrilotriacetic acid (NTA), another strong complexing agent, which exhibits a significant pH dependency, ethylenediaminetetraacetic acid (EDTA), the chelating agent used in this study, is generally pH insensitive and can remain relatively constant over a broad pH range (4.9 - 11.3) as demonstrated by Peters and Shem [28]. Cadmium, a highly toxic, non-essential (HM) pollutant is generally more mobile than lead within plant tissues and therefore, is capable of exerting more toxic effects. Toxicity will generally manifest itself in visible attributes such as wilting, and early induced senescence. None of these characteristics were apparent in wheat plants during this study period. Shoot biomass showed an interesting trend as well. Biomass is an important factor since the success of a phytoremediation process is dependent on adequate plant yield and high metal concentration in plant shoots. Plants must therefore produce sufficient biomass while accumulating high concentrations of heavy metals [29]. Figure 1 shows wheat shoot biomass grown in various Cd treatments with and without EDTA and harvested at 30, 45, and 60 days after emergence. Shoot biomass increased over time with the highest biomass being observed at day 60 with treatments of 100, 250, and 500 ppm Cd without EDTA. The lowest biomass was seen in treatments of 500 ppm Cd plus EDTA and more pronounced during the last two harvesting periods. We concluded that the toxicity of Cd at 500 ppm may have stunted the growth of Triticum aestivum L. cv. TAM-109 though not actually causing death of the plant. These results were also reported by Miller and her colleagues [30] in a greenhouse study. These investigators found that when Sesbania exaltata plants were allowed to grow in a greenhouse for 6, 8, and 10 weeks, they reported an increase in both root and shoot biomass in week 8 for plants grown in Pb-spiked soil as compared to the control. This observation was contrary to a decrease in biomass due to the plant's natural response to toxic effects of Pb. Moreover, they attributed their observation to Sesbania's ability to not only withstand elevated levels of Pb but to perhaps utilize the nitrate in [Pb(NO3)2] as a nutrient source for a limited period of time [30]. Cadmium concentrations in wheat shoots were highest during the first harvesting period and in treatments of 250 and 500 ppm, respectively (Figure 2). After 45 days wheat Cd shoot concentrations were higher in 250 and 500 ppm treatments amended with EDTA, as compared to 250 and 500 ppm without the addition of EDTA. By day 60, Cd concentrations in wheat shoots were somewhat stabilized, with the highest Cd seen in 500 ppm plus EDTA. While wheat continued to uptake Cd, especially at the highest treatment levels, it was not recognized as a metal hyperaccumulator. Hyperaccumulators are plants species that are capable of accumulating metals at levels 100-fold greater than those typically measured in shoots of the common nonaccumulator plants. Thus, a hyperaccumulator will concentrate more than 10 ppm Hg, 100 ppm Cd, 1000 ppm Co, Cr, Cu, and Pb; 10,000 ppm Zn, and Ni. To date, approximately 400 plant species from at least 45 plant families have been reported to hyperaccumulate metals [31]. Most of these species accumulate Ni, about 30 accumulate either Co, Cu, and Zn, even fewer accumulate Mn.
4. Conclusions
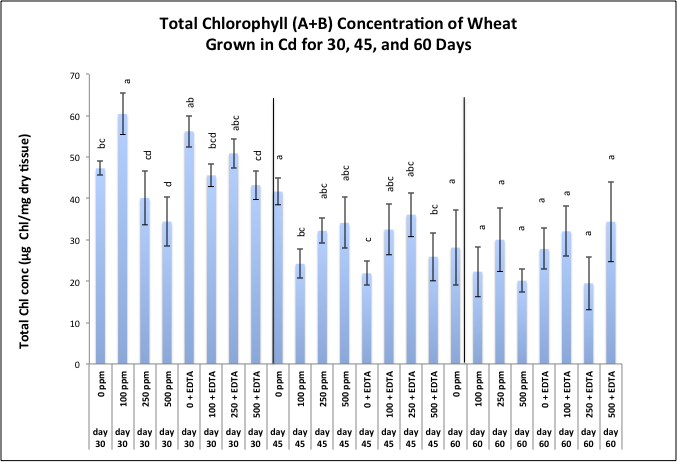
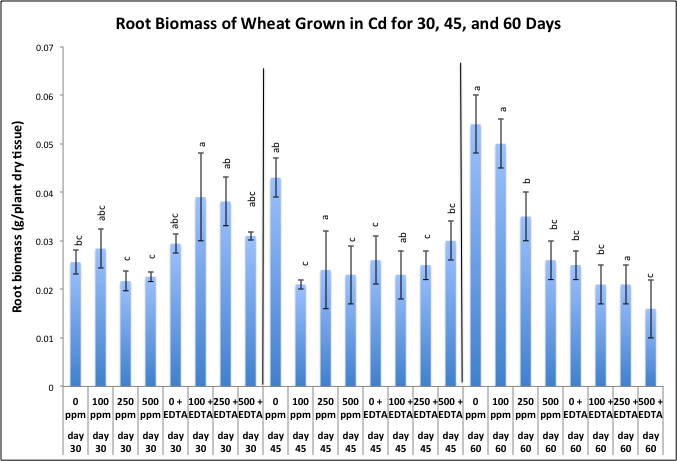
- Chlorophyll production decreased over time, and showed a slight increase with the addition of EDTA. We conclude from this preliminary study that chelates may have not only enhanced the uptake of Cd, but the chelate may have also sequestered the metal in a way that protected the plant from some of the toxic effects thereby promoting continued chlorophyll production under toxic conditions. The success of phytoextraction process depends upon both shoot biomass and shoot metal concentration. Therefore, the potential effectiveness of plants for phytoremediation is evaluated by calculating the metal accumulation inside the plant (metal concentration x plant dry weight). The heavy metal removal by plant shoots is an important index which is useful for the practical application of treatments. Our results show that there were significant differences in the uptake of Cd exhibited by wheat plants exposed to various Cd treatments. One of the first requisite in phytoextraction is the production of high plant biomass at the contaminated site. Metal phytotoxicity causes stress to the plant, resulting in a reduction in biomass and in some cases eventually death. Generally, T. aestivum produced a high biomass and was tolerant to elevated levels of Cd/EDTA in the soil. Our results further support findings by other investigators that have reported the amounts of heavy metal absorbed by wheat was higher in the roots as compared to the amount of metal translocated to shoots. It is also generally agreed that chelating agents can form strong complexes with some metals. The efficacy of the chelate to remobilize these heavy metals from a contaminated soil depends on the association of the metal with the soil matrix, as well as the chemistry of the metal. In this context, the total concentration of the metal present in the polluted soil provides little insight into the solubility and/or extractability of the metal. Rather, it is the knowledge of metal speciation in the soil that is essential in predicting the outcome of the phytoextractive process. The selection of phytoremediating species is possibly the single most important factor affecting the extent of metal removal. Although, the potential for metal extraction is of primary importance, other criteria, such as ecosystem protection must also be considered when selecting remediating plants. As a general rule, native species are preferred since exotic plants can be invasive and can endanger the harmony of the ecosystem [38].Another important factor is the complex interactions such as soil pH, location, and extent of contamination, that take place under site-specific conditions require that metal phytoextraction must be approached as a multi-disciplinary research effort incorporating the expertise of plant biologist, soil microbiologists, agronomists, and environmental engineers to identify and solve issues such as 1) how to best enhance Cd bioavailability for uptake while avoiding groundwater contamination, 2) means of optimizing the plant's ability to take up and store Cd, and 3) the use of Chl production as a biomarker for HM toxicity in plants as well as ways by which Chl production may be employed as a mechanism for increasing plant biomass. These and other creative research tools may provide a means of cleaning up metal contaminated soils that far supersede the possibilities of phytoextraction. It is therefore likely that further technical refinements are needed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML