-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2015; 5(4): 135-139
doi:10.5923/j.env.20150504.01
A Survey of the Level of Bisphenol A (BPA) in Effluents, Soil Leachates, Food Samples, Drinking Water and Consumer Products in South-Western Nigeria
Makinwa T. T., Uadia P. O.
Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Correspondence to: Makinwa T. T., Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Bisphenol A (BPA) is one of the highest volume chemicals produced. Human exposure to BPA is continuous because of its use in the production of polycarbonated plastics, and as an intermediate and additives for the production of other products. These products leach detectable levels of BPA which has found its way into our foods and drinks; these have been the major sources of human exposure to BPA. BPA is also released into the environment through sewage, industrial effluent, treatment effluent, landfill leachates or natural degradation of polycarbonate plastics. This study was carried out to determine the level of environmental and human exposure to BPA in two south western states of Nigeria. The indirect competitive enzyme-linked immunosorbent assay (ELISA) method was used for the determinations. The limit of detection (LOD) is 5ng/ml. The environmental levels were investigated in soil leachates, rivers and industrial effluents (n=9), while the sources of human exposure were investigated in canned food and drinks (n=5), and water storage containers (n=15) household materials (spoons, biros, tooth brush, phone casing and kettles) (n=7). The results confirmed that BPA was present in all the samples in a range of 96.50±1.19µg/L-2880.50±8.35µg/L. This study revealed that leaching of BPA from drink and food cans as well as consumer products are some of the major sources of human exposure and that BPA is continually released at environmentally relevant concentrations in the south western part of Nigeria through industrial effluent, dumpsite leachates, creating challenges for remediation.
Keywords: Bisphenol A, Environmental level, Sources of Human exposure, South-Western Nigeria
Cite this paper: Makinwa T. T., Uadia P. O., A Survey of the Level of Bisphenol A (BPA) in Effluents, Soil Leachates, Food Samples, Drinking Water and Consumer Products in South-Western Nigeria, World Environment, Vol. 5 No. 4, 2015, pp. 135-139. doi: 10.5923/j.env.20150504.01.
Article Outline
1. Introduction
- Bisphenol A (BPA) is one of the highest volume chemicals produced worldwide, with over 6 million pounds produced each year [1]. BPA is used as a monomer in the production of plastics [2], and as the base compound in the manufacture of epoxy resins, printer ink, automobile and optical lenses, carbonless papers, adhesives, powdered paints, dental sealants and composites [2, 3]. Studies revealed that these products leached detectable levels of Bisphenol A [3-5]. Moreover high level of BPA has been found in underground water, rivers, streams, landfill leachates [6] air and dust [7], drinking water and in food samples [8]. Hence, the population is directly and chronically exposed to BPA through consumption due to its use in food and beverage packaging and storage containers [5] as well as drinking water. The potential for BPA exposure was demonstrated when BPA was detected in 95% of urine samples in the USA. BPA has also been detected in other human body fluids ranging from 0.2 to 1.6ng/ml in serum and 0.2-20ng/ml in amniotic fluid, neonatal blood, placenta, cord blood and human breast milk [9]. Data from multiple sources indicated that the amount of BPA to which humans are exposed may cause adverse health effects [10]. This has raised concerns among regulatory agencies all over the world because of its estrogenic properties in vitro and in vivo and the conserved role that estrogen plays in regulating human and animal physiology and pathophysiology [10, 11]. Indeed, it has been suggested that exposure to xenoestrogens such as BPA during early development may be a major contributing factor to the increased incidence of infertility, genital tract abnormalities, obesity, attention deficit hyperactivity disorder, infertility, and prostate and breast cancer observed in European and U.S. human populations over the last 50 years [12]. A study by Hong et al., revealed there is a positive association between urinary BPA and oxidative stress in humans [13]. Another study by Yang et al., found that total BPA levels were associated with markers of stress in postmenopausal women [14]. In addition, BPA levels in blood have been associated with a variety of conditions in women, including obesity, endometrial hyperplasia, endometriosis, sterility, polycystic ovary syndrome (PCOS) [15] and recurrent miscarriage [16]. Even more recent studies have identified relationships between BPA exposure and reproductive hormone levels in male patients at an infertility clinic [17] and the number of oocytes retrieved from women undergoing IVF fertility treatments [18]. It is therefore expedient to carry out a bio-monitoring study on the environmental level of BPA by determining its levels in effluents, soil samples and Nigerian-made consumer products across south-western Nigeria.
2. Materials and Methods
2.1. Reagents/Chemicals
- Pre-coated Bisphenol A detachable coupling antigen ELISA micro plate: (96 wells) obtained from Ausmaucos Pharma Australian Co. Ltd, China.
2.2. Methods
2.2.1. Experimental Design/sources of Samples
- Group 1- Effluent samples were obtained from 3 plastic companies in Lagos State Nigeria and water samples were obtained from 2 dumpsite rivers in Lagos State, Nigeria and Owo, Ondo State, Nigeria as shown in figure 1 and bore hole water was also used as the control in this group. Three different soil samples were collected from these locations.
 | Figure 1. Picture showing a dumpsite River at Owo Town, Ondo State, Nigeria, where samples were collected at different points |
2.2.2. Sample Preparation
- Samples were collected in pre-cleaned glass bottles with aluminium foil-lined caps and stored under room conditions. The samples were subjected to analysis within two days to avoid degradation or transformation of native samples. (1) Soil samples (10g) were placed in a homogeniser, and 20ml of distilled water was added, mixed thoroughly, centrifuged at 1000 r.p.m for 3min and filtered, the supernatants obtained were used for further analysis. The effluent samples were mixed separately and centrifuged at 1000 r.p.m 3mins; the supernatants were used for further analysis.(2) Solid samples (10g) were placed in a homogeniser, 20ml 70% methanol solution was added, mixed thoroughly and centrifuged at 1000 r.p.m 3mins; the supernatants were filtered with Whatman No 1 filter paper. 400μl of sample diluents was added to 100μl treated sample supernatant, from which 50µl was used for analysis (dilution ratio 1:10) (3) All liquid samples were not pre-treated. The BPA levels were determined directly. Analysis was carried out in duplicate.
2.2.3. Quantitative Determination of BPA in Samples
- The BPA concentrations were determined using: Bisphenol A (BPA) ELISA Kit (obtained from Ausmaucos Pharma Australian Co. Ltd, China). This kit uses competitive ELISA method, in which the microplate is coated with Bisphenol A coupling antigen and the Bisphenol A standard or sample was added to compete with anti Bisphenol A antibody conjugates. It uses TMB chromogenic substrate, after addition of the Stop Solution, the colour will turn from blue to yellow, which can be detected at 450nm wavelength with a microplate reader. The light absorption value and Bisphenol A content in samples are inversely proportional. The Bisphenol A content in samples was calculated using a standard curve of various Bisphenol A concentrations.
2.3. Statistical Analysis
- Values are expressed as the mean ± SD. Results were statistically analyzed by one-way analysis of variance (ANOVA) for differences between means of different groups. All data were analyzed using SPSS statistical package (SPSS Inc.) version 13.0.
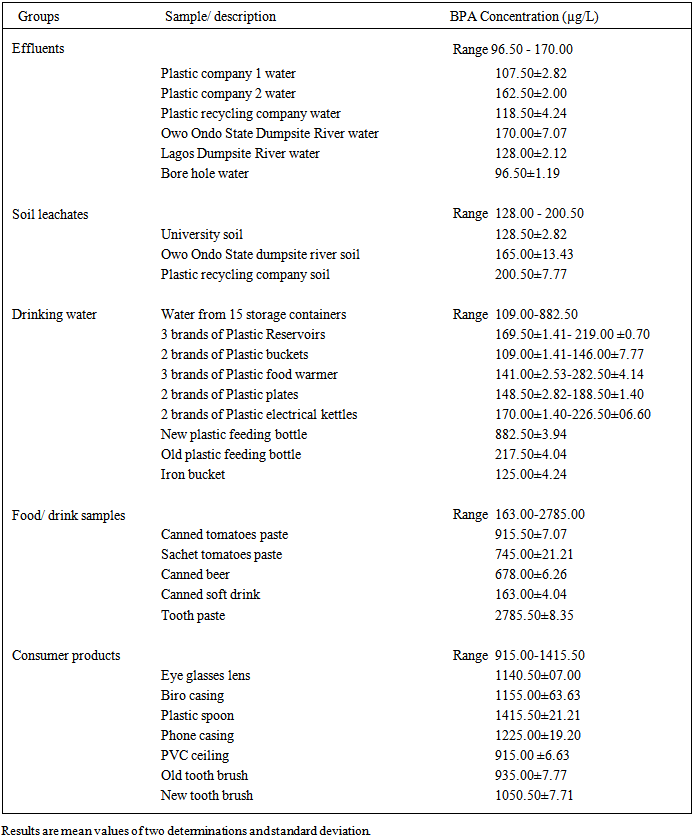
3. Results
- Table 1: Shows the concentration of BPA in different effluent samples (from different locations in Lagos and Ondo States), soil leachate samples (from different locations), water samples obtained from borehole and water samples stored in different plastic containers and metal container, beverage, food and paste samples from canned drinks and resin coated sachets, and leachate samples from consumers products obtained from Owo in Ondo State.
|
4. Discussion
- Bisphenol A (BPA) is predominantly an intermediate for the production of other products its main uses include; binding, plasticizing, and hardening functions in plastic products, paints/lacquers, binding materials and filling-in materials. The substance is used in the chemical industry, the iron/metal industry, the building and construction industry, the plastics industry and the service industry [19]. Among the 230 chemicals considered to be endocrine disruptors [20], BPA has drawn a lot of considerable attention with respect to its estrogenicity and because of its high potential for human exposure. High levels of BPA are also found in the environment and this is one of the major concerns for regulatory bodies all over the world. As shown in Table 1, the water samples stored in different plastic containers contained very high levels of BPA (152-798µg/L) compared with the water sample stored in iron container (122µg/L). Table 1 also showed the level of BPA in the various beverages (180µg/L and 667µg/L), tomatoes paste (760µg/L and 960µg/L) and toothpaste (2880µg/L) samples. The results indicate the presence of significant amount of BPA in both water samples and food samples. Human exposure to BPA occurs primarily via hydrolysis of polycarbonate plastics and epoxy resins, resulting in low concentrations of free BPA in food and liquids [21]. This makes dietary consumption the major mode of human exposure [22]. The result also revealed that significant amount of BPA leached from the consumer products (Eye glasses lens, Biro casing, Plastic spoon, Phone casing, PVC ceiling) studied (960µg/L-1400µg/L). While the source for human exposure to BPA is food and liquid storage containers, other sources of human exposure to BPA are due to increased utilisation, distribution and abundance of plastics and plastic material in the environment as well as the broad applications and the persistence of plastic materials in the environment.However, BPA enters the environment by way of wastewater, primarily through discharge into water bodies, [19] sewage treatment effluent [23], landfill leachates (via hydrolysis of BPA from plastics [24], or natural degradation of polycarbonate plastics. A large proportion comes from businesses that produce and process Bisphenol A. At the top of the list are manufacturers of polycarbonates and epoxy resins which releases BPA to the environment mainly from wastewaters and washing residue produced during production and processing of products, fugitive dust freed during handling and minor volatilization losses during manufacturing [19]. Table I showed the significant levels of BPA in the effluents collected from the plastic manufacturing and plastic recycling companies (121µg/L-234µg/L) compared with the level of BPA in the water sample drawn from bore-hole (103µg/L). BPA may enter the environment through possible physical and chemical breakdown during disposal and recycling operations in plastic recycling process. As shown in the table the level of BPA in the river water obtained at two different dumpsites revealed that BPA is present in a significant (176µg/L and 203µg/L) amount when compared with that obtained from bore-hole. The table also showed the level of BPA in the soil leachates is also significant (122µg/L-726 µg/L). Although there is a significantly higher level in those obtained from the plastic recycling company compared with that obtained from the water bed at the dumpsite. This may be due to the aerobic condition of the wash off by the water current. Rivers, lakes, and estuaries are major sinks for BPA. These surface waters accumulate BPA leached from plastic debris and landfill wastes along with BPA-containing sewage and effluent. In aerobic environments such as most rivers, BPA has an environmental half-life of between 4 and 7 days [25], being degraded primarily by bacteria [26]. However, BPA has limited biodegradation under anaerobic conditions [27], leading to concerns about BPA accumulation in anaerobic sediments of habitats such as estuaries [28]. Furthermore, companies that produce and recycle thermal paper or process PVC plastics discharge Bisphenol A into the environment. Sewage effluent and landfill leachates are point sources of BPA in the environment, fragments of epoxy resins and polycarbonate plastic debris entering the watershed through runoff are non-point sources.
5. Conclusions
- In conclusion this study revealed that leaching of BPA from drink and food cans as well as consumer products such as storage tanks, feeding bottles, plates, tooth pastes and tooth brush are some of the major sources of human exposure. The study also revealed that BPA is continually released into the environment (soil, aquatic environment) at environmentally relevant concentrations in the south western part of Nigeria through industrial effluent, dumpsite leachates creating challenges for remediation and alternative sources of raw materials in place of BPA, while additional studies are needed to examine other potential sources and routes of exposure to BPA.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML