-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2015; 5(2): 80-89
doi:10.5923/j.env.20150502.06
Potential Toxic Metals Concentrations in Different Aquatic Lives Harvested from Lagos Island and Epe Lagoons
Rasaq A. Olowu1, Abayomi A. Jimoh2, Chionyedua T. Onwordi1, Olumayowa J. Onipede3, Babajide A. Ogunnaike1, Albert O. Amosu4
1Department of Chemistry, Lagos State University, LASU Post Office, Lagos, Nigeria
2Department of Fisheries, Lagos State University, LASU Post Office, Lagos, Nigeria
3Department of Chemical sciences, Bell University of Technology, Ota, Nigeria
4Department of Agricultural Science, School of Vocational and Technical Education, Adeniran Ogunsanya College of Education, Festac town, Nigeria
Correspondence to: Rasaq A. Olowu, Department of Chemistry, Lagos State University, LASU Post Office, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The level of some Potential toxic metals PTM (Zn, Fe, Mn, Cu, Pb and Cd) in Synodontismembrane (Catfish), Tilapiazilli (Tilapia), Callinectesamnicola (Crab) and Macrobrachiummacrobachion (Prawn), from Lagos Island and Epe lagoon were investigated using Atomic Absorption Spectrophotometer (AAS), model Buick Scientific 210 GVP, the analysis on all the samples were done in triplicates. The analysis of PTM in Synodontismembrane and Tilapiazillii was conducted on head, gills and trunk, and the results indicated the accumulation of metals differently in various parts of the fishes. Lead (Pb) and Cadmium (Cd) were not detected (ND) from all the samples, this may be due to the existence of few or no industrial activities around the locations. The orders of accumulation in Catfish were Head>Gill>Trunk and the pattern of distribution is Zn>Fe>Mn>Cu in all the organs. In Tilapia, the accumulation follows the order; Gills (Fe>Zn>Mn>Cu)>Head (Zn>Fe>Mn>Cu)>Trunk (Fe>Zn>Mn>Cu). The mean values of the Crab obtained fell in the ranges: Fe (19.7±1.7- 24.7±7.7); Zn (8.0±2.5-11.6±1.3); Cu (9.0±3.3-10.3±1.9); Mn (5.0±2.9-7.3±3.7). While that of the Prawn falls in the ranges: Fe (6.3±1.9-4.0±1.6); Zn (6.3±2.9-8.0±2.5); Cu (2.0±1.0-2.7.±0.9); and Mn (1.7±0.5-3.0±0.8) for Lagos Island and Epe Lagoon respectively. The high concentrations of iron and zinc in the fish parts could be associated with the fact that these metals are naturally abundant in Nigerian soils and since the source of metal depositories are the aquatic systems. The general trend of accumulations in all the organisms were Chrysichthysnigrodigitatus>Tilapiazillii>Callinectes,amnicola>Macrobrachiummacrobrachion. The analysis of the Crab and Prawn shows that concentrations of Zn and Fe in the samples are lower than the permissible limits set by WHO and FEPA, while Mn and Cu concentrations in the fish samples were above the standard set limits hence consumption of the fish from the analysed lagoon may be dangerous to health, therefore it is pertinent to regularly monitor the activities of both the lagoons.
Keywords: Persistent, Human consumption, Fish part, Deposition, Aquatic organisms, Bioaccumulation
Cite this paper: Rasaq A. Olowu, Abayomi A. Jimoh, Chionyedua T. Onwordi, Olumayowa J. Onipede, Babajide A. Ogunnaike, Albert O. Amosu, Potential Toxic Metals Concentrations in Different Aquatic Lives Harvested from Lagos Island and Epe Lagoons, World Environment, Vol. 5 No. 2, 2015, pp. 80-89. doi: 10.5923/j.env.20150502.06.
Article Outline
1. Introduction
- Recent studies have shown for instance that human activities have created ecological pressure on the natural habitat of fish and other marine organism over time. The diverse groups of animals that leave and grow in water are said to be aquatic animals. An incredible variety of species lives in the different parts of these environments [1]. It is impossible to overstate the importance of these aquatic lives to human populations around the world. [1-2]. There is an upsurge of interest in water pollution as a result of its deleterious effect. Moreover, factors such as high population growth accompanied by intensive urbanization, increase in industrial activities and higher exploitation of natural resources including cultivable land have resulted in pollution increase [3]. There has been a steady increase in discharges that reaches the aquatic environment from industries. Huge amount of organic material are discharge into the water body even though some industrial process for instance pump mill and sugar processing plant also generate much finely divided organic material as waste product, which is broken down easily by bacteria activities resulting in the reduction of oxygen level or even anaerobic condition in the vicinity of an effluent [1]. Moreover, to direct depletion of oxygen the decomposition of large quantities of organic material in the water produces inorganic nutrients such as ammonia, nitrate and phosphorus [3]. These enrich the water significantly resulting in dense algae growth or bloom which can cause the wide daily fluctuations in oxygen described for fish pound and in extreme condition, fish–kill can result [2-3]. However the increased productivity caused by excessive organics load can cause a decline in water quality [4-6]. Fish are often at the top of the aquatic food chain and may concentrate large amounts of some metals from the water [1-3]. Besides, fish is one of the most sensitive indicators of trace metals pollution and risk potential of human consumption [7]. Aquatic foods have essential amino acids, fatty acids, protein, carbohydrates, vitamins and minerals. Among sea foods, fish are commonly consumed and hence, are a connecting link for the transfer of toxic heavy metals in human beings [1-2, 8]. PTM have the tendency to accumulate in various organs of marine organisms, especially fish, which in turn may enter into the human metabolism through consumption causing serious health hazards [2, 9]. Crab is a common name for any of a group of crustaceans characterized by a reduced abdomen and an enlarged and broadened anterior portion of the body and is most common as bottom dwellers in the sea [9-10]. Crabs often reach a considerable size the largest being the spider crab, with long, thin legs spanning up to 3.6 m (up to 12 ft) and they possess fairly complicated nervous systems. They are able to resist changes in the external environment and flourish in rather hostile habitats and their food habits varied. Crabs have compound eyes and can see well and their senses of smell and taste are also well developed as well as allow them to identify both food and prospective mates [9-10]. Generally, crabs are edible, and their meat is rich in protein and low in fat and they tend to be aggressive toward one another, and the males often fight to gain access to the female. For a commercial fishery to succeed, the crabs must be abundant and cheaply caught, usually in “pots” or with nets. At the present time, because of overfishing, pollution, and the cost of energy, the prospect is small for increasing the yield of these types of fisheries [6]. Prawns belong to the order Decapoda., the common European prawns, classified as Palaemonserratus, and an edible prawn found off the Atlantic coast of America, classified as Palaemonetesvulgaris, belong to the family Palaemonidae [9-11]. The southern species of edible prawn often marketed as shrimp belongs to the family Penaeidea and is classified as Panaeussetiferus .Prawn is a common name applied to numerous species of shrimplike, ten-legged crustaceans. The prawn is distinguished from the shrimp mainly by a long serrated rostrum or beak that projects from the shell. Prawns are widely distributed in both fresh and brackish waters in temperate and tropical regions [11]. Both crab and prawn have been reported as bottom feeders and also accumulate heavy metal over a period of time [9]. In small quantities, certain PTM are nutritionally essential for a healthy life [12-15]. These elements, or some form of them, are commonly found naturally in foodstuffs, in fruits and vegetables, and in commercially available multivitamin products [15]. Some are used in diagnostic medical applications such as direct injection of gallium during radiological procedures, the use of lead as a radiation shield around x-ray equipment and the use of silver and mercury amalgam for tooth filling [16-17]. Essential PTM also have normal physiological regulatory functions. PTM are also common in industrial applications such as in the manufacture of pesticides, batteries, alloys, electroplated metal parts, textile dyes, steel, catalysis and so forth [15-20]. Trace metals like lead, cadmium, manganese and zinc have been classified as neurotoxic metals to children [21-25]. Conversely, essential PTM become toxic when their amount exceeds those required for correct nutrition [17-18, 26-30]. For some PTM, toxic levels can just be a little above the background concentrations naturally found in nature. Long-term exposure to PTM may result in slowly progressing physical, muscular, and neurological degenerative processes. Allergies are also common and repeated long-term contact with some metals or their compounds may even cause cancer and eventual death [2, 15, 31-33]. The present research work is geared toward ascertaining the level of PTM in the selected samples (Tilapia, Catfish, Prawn, and Crab) harvested from Lagos Island and Epe Lagoon.
2. Materials and Methods
2.1. Study and Sampling Area
- The samplings were conducted on two water bodies, Epe Lagoon and Lagos Island Lagoon. Epe Lagoon is located in Lagos State, an African megacity which is located in south-western Nigeria on the West Coast of Africa, within latitudes 6o 23’N and 6o 41’N and Longitudes 2o 42’E and 3o 42’E. Lagos Island Lagoon on the other hand is also located in Lagos State. Lagos Island is a Metropolitan area and is located on geographic grid reference Latitude 60 27’N, Longitude 3024’E. The samples were harvested from the downstream of Lagos Lagoon at a specific site which is popularly called Gbobaniyi market (6°27’N and Longitude 3°23’E) and the downstream of Epe Lagoon (Latitude 6°27’N and Longitude 3°23’E). The samples selected for analysis were: Catfish, Crab, Prawn and Tilapia Fish. These are commercially important and nutrient aquatic animals.
2.2. Sampling Methods and Preparation
- (a) Tilapia and Catfish Sampling and PreparationThe Tilapia Fish and Catfish were identified as Tilapia zilli and Synodontis membrane respectively at the department of Fisheries Lagos State University, they were collected from commercial fishermen in downstream of Epe and Lagos island Lagoon and were kept in sterile polythene bags, and taken in ice box to the laboratory where they were washed with running tap water to remove dirt and kept in the laboratory deep freezer to prevent deterioration till further analysis. For analysis, the fish samples were defrosted for two hours. The scales were removed and each separated into Head, Gill, and Trunk using a plastic knife. The fish parts from the two lagoons were dried at 80oC for 2 hours in Gallenkamp hot box oven and then blended in a wood made mortar and pestle. Approximately 2.0 g each of sample was weighed and ashed in the furnace at 550oC for 90 minutes. The ash was dissolved in 5 mL of concentrated nitric acid and made up to 25 mL volume [2, 16]. Atomic Absorption Spectrophotometer (AAS), model Buck Scientific 210GVP was used to determine the presence of Zinc, Cadmium, Copper, Manganese, Lead and Iron.(b) Prawn and Crab Sampling and preparationThe Prawn and Crab samples identified as Macrobrachium macrobachion and Callinectes amnicola respectively were purchased from the divers at the downstream of the Lagos island and Epe Lagoons and placed into an ice-cooled container, each prawn and crab samples was properly cleaned by rinsing with distilled water to remove debris, planktons and other external adherent. It was then drained under folds of filter, weighed, wrapped in aluminum foil and then frozen prior to analysis. The crab and prawn sample were defrosted for 2 hours, it was then weighed into a pre-weighed petri-dish, and then dried at 80oC in Gallenkamp hot box oven. The dried samples weight were taken and recorded at intervals of 4 h until a constant weight was obtained. The dried samples of crab and prawn were put in a cleaned dried mortar separately and were grounded to fine particles and then sieve using a sieve of particle size 2 mm. 0.5 g each of samples were measured into clean dried beaker (100 mL), 5 mL of aqua regia (HCl and HNO3, 3:1) was then added to the sample for digestion. The samples were allowed to be evenly distributed in the acid by stirring with a glass rod and then the beaker was place on the heater. The digested sample was filtered into a standard volumetric flask of 50 mL capacity and the filtrate was made up to mark using distilled water. Atomic Absorption Spectrophotometer (AAS), model Buck Scientific 210GVP was used to determine the concentrations of PTM in the digested samples.
3. Results and Discussions
3.1. Results
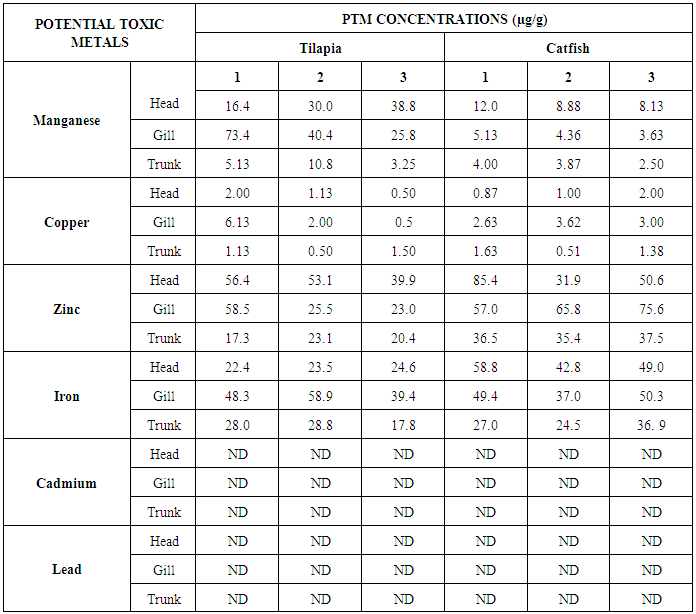
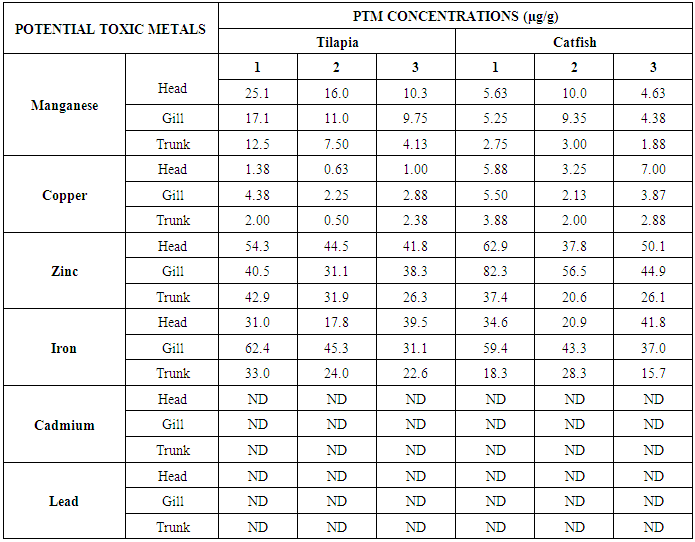
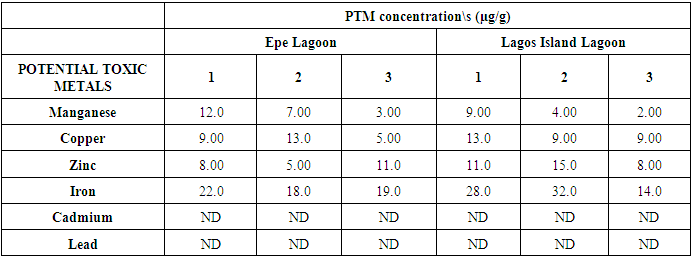
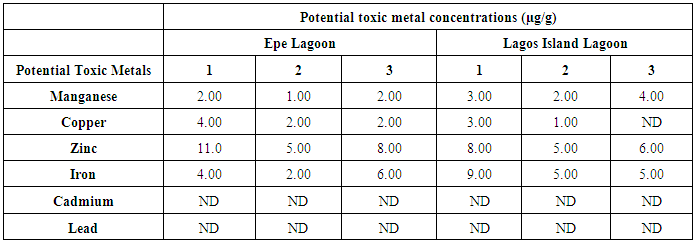
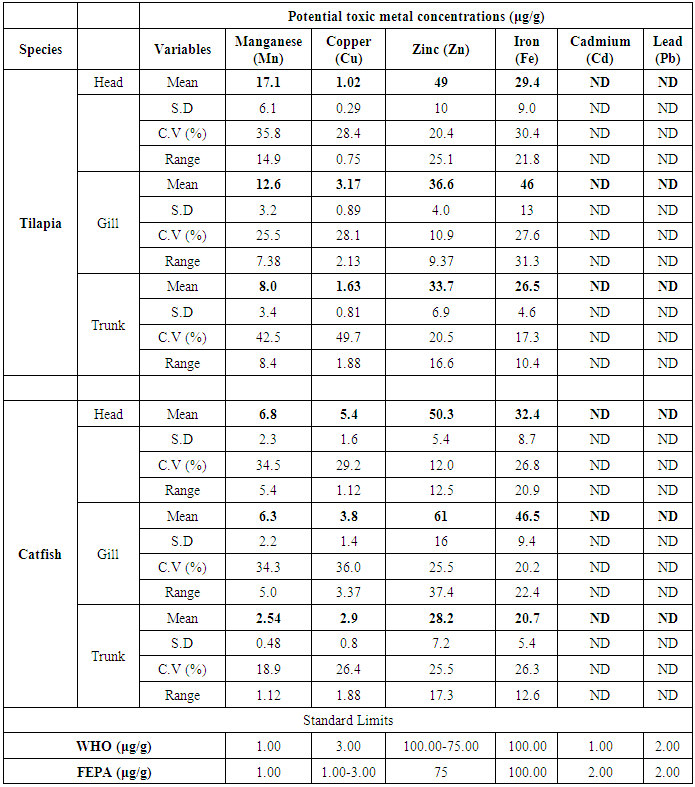
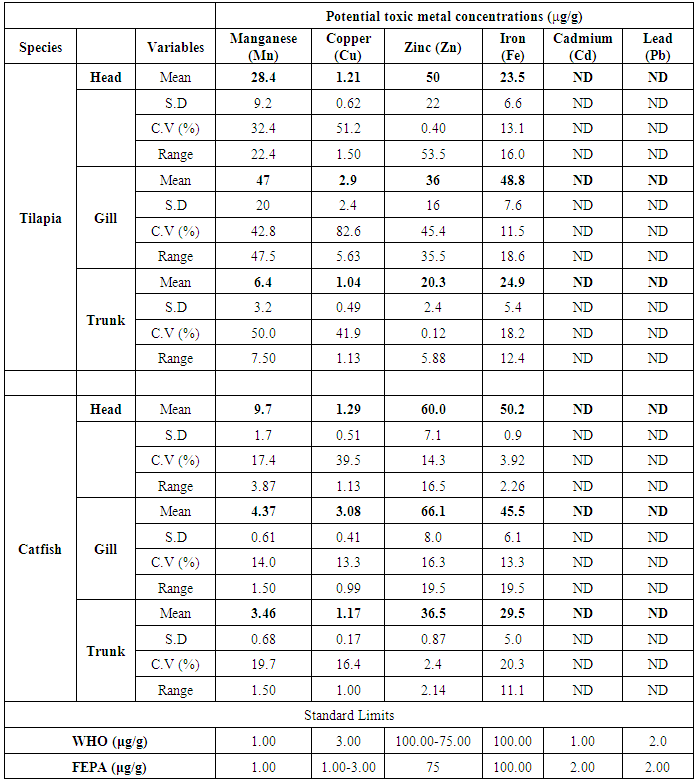
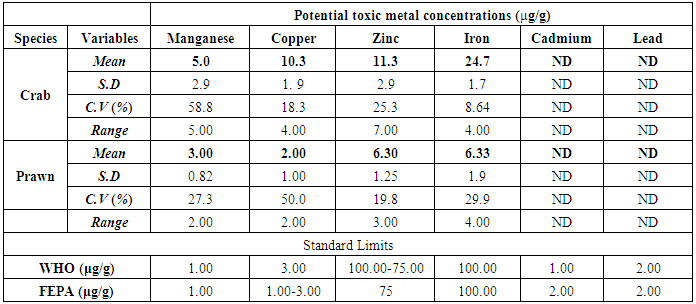
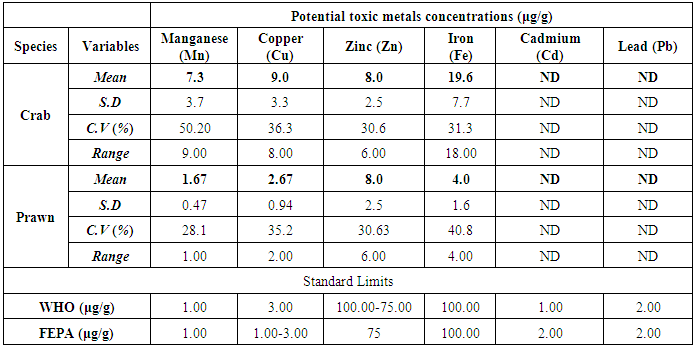
- The results obtained from Catfish and Tilapia fish samples collected from Lagos Island and Epe lagoon for the concentration of cadmium (Cd), manganese (Mn), iron (Fe), lead (Pb), and zinc (Zn) are summarized in Tables 1A and 1B respectively, while, the results obtained for the crab and prawn are summarized in Tables 2A and 2B respectively.
|
|
|
|
3.2. Discussions
- In the previous section it is shown that the overall concentration of metals from both fish species were slightly more (although not in all cases) in Lagos lagoon than from Epe lagoon, this may not be farfetched from the fact that the Lagos lagoon is situated in a more developed (urbanized) area with high industrial activities and the lagoon may be prone to more effluent discharge than the Epe lagoon. Apart from manganese level in both fish species it could be noticed that other PTM (Zn, Cu, Fe) indicated higher concentrations in the Catfish than Tilapia, this might be attributed to the difference in nature of skin but the concentration level were below allowed permissible level, though Pb and Cd were not detected in the samples from both locations while tilapia has scales on the body, the catfish is smooth and slimy this is in agreement with earlier report [1-3]. It was also discovered that in the two fish species, the metal concentrations in the gills were mostly higher than those in the head and trunk as shown in the table indicating that the metal uptake was mostly through gills absorption. Thus, the concentrations of metals in gills reflected the concentrations of metals in the water, where the fish species lives which was in agreement with earlier report (1, 34).The concentration of Zinc was found to be the highest in the fish species obtained from both Lagos Island and Epe lagoon as shown in Table 3A and 3B respectively which is followed by Iron, Manganese and Copper i.e. Zn>Fe>Mn>Cu with absence of lead and cadmium in all the sample analyzed. The concentrations of Mn in both samples harvested from Epe and Lagos Island lagoon were above the standard values set by WHO and FEPA [35-36]. While Cu concentration was between the limit in both samples from the two locations. Zn and Fe concentrations were very much below the standard limits which agree with earlier report (1-2, 34). The values of heavy metals obtained in the prawn from both locations are lower than the maximum permissible limits set by the World Health Organization (Fe = 100 µg/g; Cu = 30 µg/g; Zn = 10.75 µg/g; Pb = 2 µg/g) and except for Cu, the values were also below the FEPA limits (Cu = 1.3 µg/g;Fe = 100 µg/g; Zn = 75 µg/g). Pb and Cd were not detected from both sampling locations. The implication of this finding is that the consumption of Macrobrachium macrobachion from both Epe Lagoon and Lagos lagoon is still safe but regular monitoring (annual) should be done to promptly detect sudden increases in these metals, especially for Pb, which causes mental retardation in children [30-32].
|
|
|
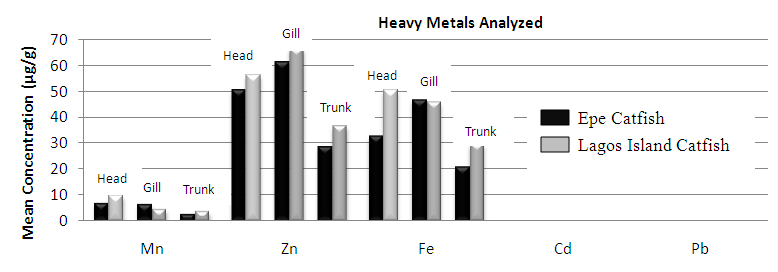 | Figure 1. Comparison of potential toxic metal concentration in catfish harvested from Lagos island and Epe lagoons |
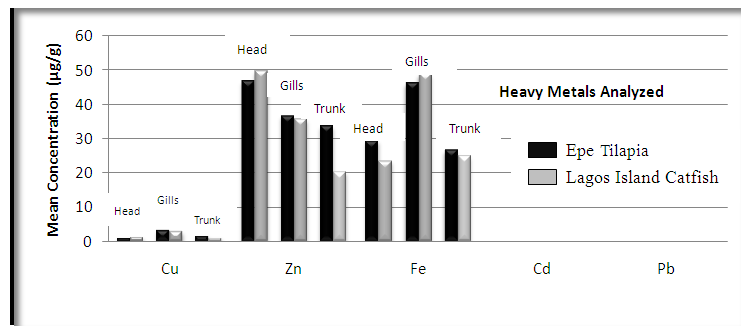 | Figure 2. Comparison of potential toxic metal concentration in tilapia from Lagos island and Epe lagoon |
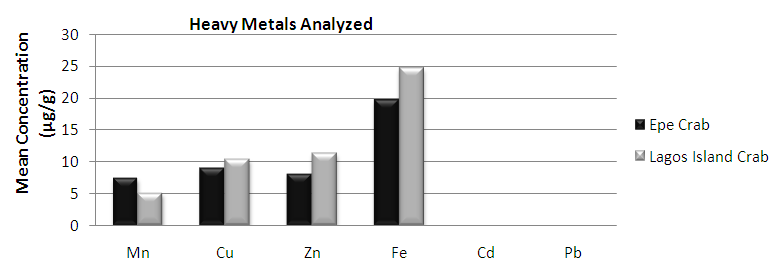 | Figure 3. Comparison of potential toxic metal concentration in crab from Lagos island and Epe lagoon |
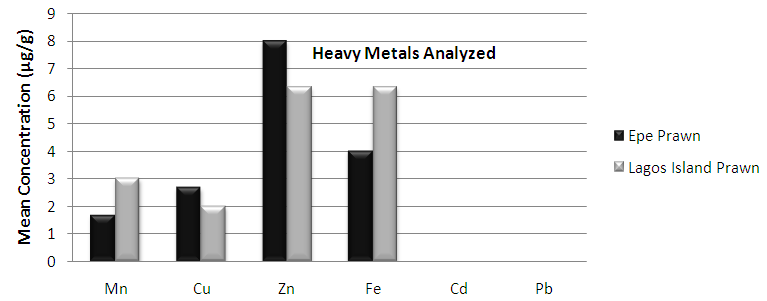 | Figure 4. Comparison of potential toxic metal concentration in prawn from Lagos Island and Epe lagoon |
4. Conclusions
- The result revealed that the different aquatic live investigated accumulate Cu, Zn, Fe and Mn, with high metal accumulation recorded in crab but the levels of PTM in crabs and prawns were below the maximum permissible limit set by WHO in foods for Cu, Zn, Fe, Pb and Cd respectively. Mn concentrations were above the limits in both samples. The levels of concentrations of Zn and Fe in both Tilapia and Catfish samples were below the permissible limits set by WHO and FEPA while Cu concentration is between the limits for both Tilapia and Catfish samples hence are still safe for human consumption but regular monitoring of the lagoons is necessary (especially the Lagos lagoon) which receive effluents from nearby industries.
References
| [1] | Olowu, R. A, Ayejuyo, O.O, Adewuyi, G.O., Babatunde, A.O. Adejoro, I.A, Denloye, A.A.B, Ogundajo, A.L, 2010. Heavy metals in fish tissue, water, sediment from Epe and Badagry lagoons Nigeria E-Journal of Chemistry 7(1), 215-221. |
| [2] | Olowu, R.A., Onwordi, C.T. Denloye, A.A, Osundiya, M.O, Adebayo, N.O Owolabi, M.S.. Tovide, O.O,. Moronkola B.A. Omoyeni, O.A. Ajuwon O.R, 2012. Heavy Metals in Oreochromis niloticus (Linnaeus, 1758) (Persiformes: Cichlidae), Ictalurus punctanus (Rafinesque, 1818) (Suliriformes: Ictaluridae) and Bottom Sediments from Lagos Lagoon Proximal to Egbin Power Plant, Ijede, Ikorodu, Lagos Nigeria Research Journal of Environmental and Earth Sciences. 4 (3), 237-243. |
| [3] | Olowu R. A, Osundiya M O, Onwordi C.T, Denloye A A, Okoro C. G, 2 Tovide O O, Majolagbe A O ,Omoyeni O A Moronkola B A, 2012. Pollution Status of Brewery Sewage Sludge in Lagos, Nigeria. International Journal of Research and Reviews in Applied Sciences(IJRRAS) 10 (1) 159-165. |
| [4] | Smith, V.H.; Tilman, G.D.; Nekola, J.C. 1999, Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems - Principles, Problems, Restoration. Environmental Pollution, Volume 100, (1-3) 179-196. |
| [5] | Khalifa K.M., Hamil A.M, Al-Houni A.Q.A., Ackacha M.A.2010. Determination of Heavy Metals in Fish Species of the Mediterranean Sea (Libyan coastline) Using Atomic Absorption Spectrometry., International Journal of Pharm Tech Research,, 2 (2), 1350-1354. |
| [6] | Indrajit Sen, Ajay Shandil and V. S. Shrivastava, 2011. Study for Determination of Heavy Metals in Fish Species of the River Yamuna (Delhi) by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) Advance in Science research 2(2) 161-166. |
| [7] | Canli M. and Atli G, 2003. Rea\lationship between heavy metal concentration (Cd, CR, Pb, Fe, Zn ) level and the size of six Mediterranean fish species. Environmental Pollution, 121(1), 129-136. |
| [8] | Papagiannis, I, Kagalou, I., Leonardos, J., Petridis, D. Kalfakakou, V., 2004. Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environ. Int., 30: 357-362. |
| [9] | Olowu R.A., Ayejuyo O.O., Adejoro I.A., Adewuyi G.O, Osundiya, M.O. Onwordi, C.T, Yusuf K A and Owolabi M S (2010) Determination of Heavy Metals in Crab and Prawn in Ojo Rivers Lagos, Nigeria E-Journal of Chemistry 7(2), 526-530. |
| [10] | Karel F. Liem 2009. Professor of Ichthyology Harvard University Article on Tilapia Fish; Microsoft Encarta Premium.version16.0.0.1117. |
| [11] | Chambers, Kenneth 2009, Article on Catfish, Microsoft Encarta Premium.version16.0.0.1117. |
| [12] | Buchsbaum, Ralph, Mildred P, John P., Vicki P.., Animals without Backbones.3rd edition. University of Chicago Press, 1987. An introduction to invertebrates for both students and general readers. |
| [13] | Daffus, John H. 2002."Heavy metals" a meaningless term? (IUPAC Technical Report)" Pure and Applied Chemistry, Vol. 74, pp. 793-807. doi:10.1351/pac200274050793. |
| [14] | IOSHIC, 1999.. International Occupational Safety and Health Information Centre. Metals. In Basics of Chemical Safety, Chapter 7, 1999 Sep. Geneva: International Labour Organization. |
| [15] | Roberts, J.R 1999. Metal toxicity in children. In Training Manual on Pediatric Environmental Health: Putting It into Practice Jun. Emeryville, CA: Children's Environmental Health Network. |
| [16] | Professor Michael Horsfall Jnr, 2011, Chemistry And Heavy Metals Are Janus-Faced Inaugural Lecture Series NO. 81. |
| [17] | Feranandes, C., A. Fontainhas-Fernandes, D. Cabral and M.A. Salgado, 2008. Heavy metal in water, sedimentand tissue of Liza saliens from Esmoriz-paramoslagoon, Portugal. Environ. Mont. Ass., 136: 267-275. |
| [18] | E. Nuray and O. Ozkan, 2007. Proximate Composition and Mineral Contents in Aqua Culture Sea Bass (Dicentrarchuslabrax), Sea Bream (Sparusaurata) Analyzed by ICP-MS Food Chem., 102, 721-725. |
| [19] | Phillip L. Williams, Robert C. James, Stephen M. Roberts. 2000. principles of toxicology John Wiley & Sons, INC, New York,. pp 138-139. |
| [20] | Kellas, B., Ph.D., and Dworkin, A., N.D. 1996. Surviving the Toxic Crisis (Olivenhain, CA: Professional Preference Publishing, 187, 217, 230-34. |
| [21] | Rasaq A. Olowu, Gregory O. Adewuyi, Olumayowa J. Onipede, Oladipo A. Lawal, Owolabi M. Sunday, 2015. Concentration of Heavy Metals in Root, Stem and Leaves of Acalypha indica and Panicum maximum jacq from Three Major Dumpsites in Ibadan Metropolis, South West Nigeria American Journal of Chemistry 5(1): 40-48. |
| [22] | Censi, P., Spoto, S. E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., Di Geronimo, S. I., Punturo, R., Ottonello, D., 2006. Heavy metals in coastal water systems.A case study from the northwestern Gulf of Thailand. Chemosphere, 64: 1167–1176. |
| [23] | M. Öztürk, 2G.Özözen, O. Minareci, E. Minareci, 2009. Determination of heavy metals in fish, water and sediments of Avsar dam lake in Turkey, Iran. J. Environ. Health. Sci. Eng.,Vol. 6, No. 2, pp. 73-80. |
| [24] | Fatoki, O.S., Lujiza, N., and Ogunfowakan, A.O. 2002. Trace metal pollution in Umtata River. Water SA, 2(2), pp. 2-13. |
| [25] | Tüzen M., 2003 .Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem., 80: 119–123. |
| [26] | Yilmazet al., (2007). Yilmaz, F., Ozdemir, N., Demirak, A. And Tuna, A.L., 2007. Heavy metal levels in two fish species Leuciscuscephalus and Lepomisgibbosus. Food Chem., 100: 830-835. |
| [27] | O.A.A. Eletta, F.A. Adekola and J.S. Omotosho, 2003. Determination of concentration of heavy metals in two common fish species from Asa river, Ilorin, Nigeria, Toxicological& Environmental Chemistry, Vol. 85, No. 1–3, pp. 7–12. |
| [28] | A. B. Tabinda M. Hussien, I. Ahmed and A. Yasar. (2010). Accumulation of Toxic and Essential Trace Metals in Fish and Prawns from Keti Bunder Thatta District, Sindh. Pakistan J. Zool., vol. 42(5), pp. 631-638. |
| [29] | O, Olowoyo, J. O., Mdakane, S. T. R. and Okedeyi, O. O, Assessing the levels of trace metal from two fishspecies harvested from treated waste water stored in a manmade lake Pretoria, South Africa, African Journal of Biotechnology Vol. 11(4), pp. 838-842. |
| [30] | Romeo, M., Siau, Z., Sidoumou, Y. and Gnassia, M. B. (1999) Heavy Metal Distribution inDifferent Fish Species from the Mauritania Coast, Science of the Total Environment, Vol.232, No. 3, pp. 169-175. |
| [31] | Okoye B C O, Afolabi O A and Ajao E A,Int J Environ Studies, 1991. 37(1), 35-42. |
| [32] | Kakalu S E, Osibanjo O and Ajayi S O, Environ Int., 1987. 13, 247-251. |
| [33] | Adedeji O.B.* and Okocha R.C. Bioconcentration Of Heavy Metals In Prawns And Water From Epe Lagoon And Asejire River In Southwest Nigeria, Journal of Applied Sciences in Environmental Sanitation, 6 (3): 377-384. |
| [34] | Babatunde A. Murtala, Waidi Oyebanjo. A, Adeolu A. Akinyemi, Bioaccumulation Of Heavy Metals In Fish (Hydrocynus Forskahlii, Hyperopisus Bebe Occidentalis And Clarias Gariepinus) Organs In Downstream Ogun Coastal Water, Nigeria. |
| [35] | World Health Organisation (WHO), (2008). Guidelines for Drinking Water Quality. 3rd Edn., Health Criteria and Supporting Information. WHO, Geneva, pp: 668. Retrieved from: http://www.who.int/water_sanitation_health/dwq/fulltext.pdf. |
| [36] | Federal Environmental Protection Agency (FEPA), (2003). Guidelines and Standards for Environmental Pollution Control in Nigeria, pp: 238. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML