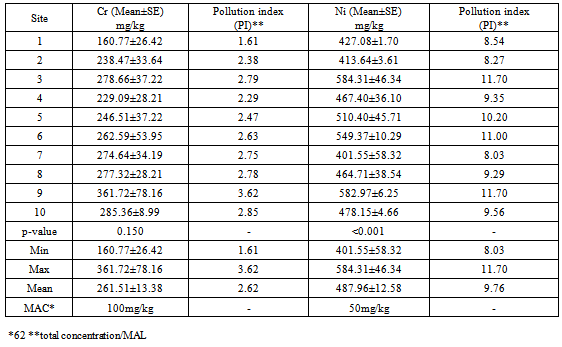
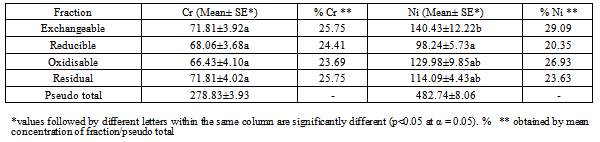
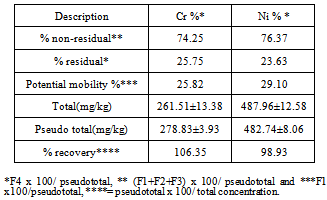
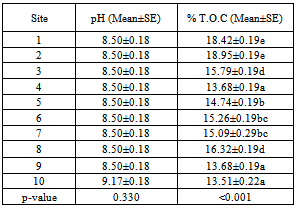
| [1] | Young, M and Crawford, J. W. (2004). Interactions and self-organization in the soil-microbe complex. Science 304: 1634–1637. |
| [2] | Kabata-Pendias, A. and Mukherjee, A. B. (2007). Trace Elements from Soil to Human.: Springer-Verlag, Berlin. |
| [3] | Alloway, B. J. (1995). Heavy metals in soils, Blackie Academic & Professional, an imprint of Chapman & Hall, London. 11-37. |
| [4] | Chen T. B., Wong, J. W. C., Zhou, H. Y., Wong, M. H. (1997).Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environmental Pollution 96: 61-68 |
| [5] | Tack, F. M. G. and Verloo, M. G. (1995). Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. International Journal of Environmental Analytical Chemistry 59: 225-238. |
| [6] | McLaren, R. G., Clucas, L. M., Taylor, M. D. and Hendry, T. (2003). Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge 1. Leaching of macronutrients. Australian Journal of Soil Research 41: 571-588. |
| [7] | McLaughlin, M. J. and Smolders, E. (2001). Background zinc concentrations in soil affect the zinc sensitivity of soil microbial processes–a rationale for a metalloregion approach to risk assessment. Environmental Toxicology and Chemistry 20: 2639–2643. |
| [8] | Speir, T. W., Van-Schaik, A. P., Percival, H. J., Close, M. E. and Pang, L. P. (2003). Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land. Water, Air and Soil Pollution 150: 319-358. |
| [9] | Zarcinas, B. A., Ishak, C. F., McLaughlin, M. J., Cozens, G. (2004). Heavy metals in soils and crops in Southeast Asia. 1. Peninsular Malaysia. Environmental Geochemistry and Health 26: 343-357. |
| [10] | Fernandes, J. C. and Henriques, F. S. (1991).Biochemical, physiological and structural effects of excess copper in plants. The Botanical Review57: 246-273. |
| [11] | Adriano, D. C. (1986). Trace elements in the terrestrial environment. Springer-Verlag New York 105–123, 533. |
| [12] | Wang, C. X., Mo, Z., Wang, H., Wang, Z. J. and Cao, Z. H. (2003).The transport, time-dependent distribution of heavy metals in paddy crops. Chemosphere 50: 717-723. |
| [13] | Lu, A., Zhang, S and Shan, X-Q. (2005). Time effects on the fractionation of heavy metals in soils. Geoderma, 125: 225-234. |
| [14] | Sharma, R. K., Agrawal, M. and Marshall, F. (2007).Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicology and Environmental Safety 66: 258 – 266. |
| [15] | Gupta, S. K., Vollmer, M.K. and Krebs, R. (1996). The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management. Science of the Total Environment. 178: 11-20. |
| [16] | Remon, E., Bouchardon, J. L., Cornier, B., Guy, B., Leclerc, J. C. and Faure, O. (2005). Soil characteristics, heavy metal availability and vegetation recovery at a former metallurgical landfill: Implications in risk assessment and site restoration. Environmental Pollution 137: 316-323. |
| [17] | Ross, S. M. (1996).Toxic metals in soil-plant system, John Wiley & Sons, Chichester, West Sussex, England. |
| [18] | Tiller, K. G. (1992).Urban soil contamination in Australia. Australian Journal of Soil Research 30: 937-957. |
| [19] | Bacon, W. G., Dalvi, A. D., Rochon, B. A. and Selby, M. (2002). Nickel outlook—2000 to 2010. Communication Bulletin. 95: 47–52. |
| [20] | Bencko, V. (1983). Nickel: A review of its occupational and environmental toxicology. Journal of. Hygiene, Epidemiology, Microbiology and Immunology 27: 237. |
| [21] | Scott-Fordsmand, J. J. (1997). Toxicity of nickel to soil organisms in Denmark. Reviews on Environmental Contamination and Toxicology 148: 1. |
| [22] | Roseby, S. J., Mulligan, D. R., Menzies, N. W., Ritchie, P. J. and Currey, N. A. (1998). Ecosystem development on tailings at Kidston gold mine, North Queensland, Australia. In: Land reclamation achieving sustainable benefits. Fox, H. R., Moore, H. M. and McIntosh, A.D., ed. Balkema, Rotterdam. 137-142. |
| [23] | Markus, J. A. and McBratney, A. B. (1996). An urban soil study: Heavy metals in Glebe, Australia. Australian Journal of Soil Research 34: 453-465. |
| [24] | Lottermoser, B. G. (1997). Natural enrichment of top soils with chromium and other heavy metals, Port Macquarie, New South Wales, Australia. Australian Journal of Soil Research 35: 1165-1176. |
| [25] | Diagomanolin, V., Farhang, M., Ghazi-Khan-Sazi, M. and Jafar-Zadeh, N. (2004). Heavy metals (Ni, Cr, Cu) in the karoon waterway river, Iron. Toxicological Letters151: 63. |
| [26] | Ayodele, J. T. and Mohammed, S. S. (2011). Speciation of nickel in Soils and Cereal. Research Journal of Applied Sciences Engineering and Technology 3: 202 – 209. |
| [27] | Bennett, B. G. (1982). Exposure of man to environmental nickel-an exposure commitment assessment. Science of Total Environment 22: 203. |
| [28] | Yaman, M., Okamus, N., Bakirdere, S. and Akdeniz, I. (2005). Zinc speciation in soils and relation with its concentration in fruits. Asian Journal of Chemistry 17: 66 – 72. |
| [29] | Lonati, G., Giugliano, M. and Cernuschi, S. (2006).The role of traffic emissions from weekends and weekday fine PM data in Milan. Atmospheric Environment 40: 5998-6011. |
| [30] | Pecheyran, C., Lalere, B. and Donald, O. F. X (2000).Volatile metal and metalloid species (Pb, Hg, Se in a European urban atmosphere (Bordeaux, France). Environmental Science and Technology 34:27-32. |
| [31] | Onianwa, P. C., Jaiyeola, O. M. and Egekenze, R. N. (2001). Heavy metal contamination of top soil in the vicinities of auto-repair workshops, gas stations and motor parks in a Nigerian city. Toxicology and Environmental Chemistry 84: 33-39. |
| [32] | Ipeaiyeda, A. R. and Dawodu, M. (2008). Heavy metals contamination of topsoil and dispersion in the vicinities of reclaimed auto-repair workshops in Iwo Nigeria. Bulletin of Chemical Society Ethiopia22:339-348. |
| [33] | Iwegbue, C. M. A. (2007). Metal fractionation in soil profiles at automobile mechanic waste dumps. Waste Management Research 25: 585-593. |
| [34] | Nwachukwu, M. A.; Feng, H. and Alinnor, J. (2010).Assessment of heavy metal pollution in soil and their implications within and around mechanic villages. International Journal of. Environmental Science and Technology 7: 347-358. |
| [35] | Adriano, D. C. (1992). Biogeochemistry of trace metals. Boca Raton, Lewis Publishers Florida 514. |
| [36] | United Nations Environmental Protection Programme (UNEP), Nairobi. (2004). Sustainable consumption and production activities in Africa: Regional status report, 2002-2004. Nairobi Kenya. |
| [37] | Akpoveta, O. V., Osakwe, S. A., Okoh, B. E. and Otuya, B. O. (2010). Physicochemical characteristics and levels of some heavy metals in soils around metal scrap dumps in some parts of Delta State, Nigeria. Journal of Applied Science and Environment 4: 57-60. |
| [38] | Iwegbue, C. M. A., Nwajei, G. E., Eguavon, O. and Ogala, J. E. (2009). Chemical fractionation of some heavy metals in soil profiles in vicinity of scrap dumps in Warri, Nigeria. Chemical Speciation and Bioavailability 21: 99-110. |
| [39] | Soon, Y. K. and Bates, T. E. (1982).Chemical pools of cadmium, nickel, and zinc in polluted soils and some preliminary indications of their availability to plants. Journal of Soil Science 33: 477-488. |
| [40] | Ahumada, I., Escudero, P., Adriana, C. M., Castillo, G., Ascra, L. and Fuentes, E. (2004).Use of sequential extraction to assess the influence of sewage sludge amendment on metal mobility in Chilean soils. Journal of Environmental Monitoring 6: 327-334. |
| [41] | Albores, A. F., Perez-cid, B., Gomes, E. F. and Lopez, E. F. (2000). Comparison between sequential extraction procedures and single extraction procedures and for metal partitioning in sewage sludge samples Analyst 125:1353–1357. |
| [42] | Norvell W. A. (1984). Comparison of chelating agents for metals in diverse soil materials, Soil Science Society of America Journal 48: 1285–1292 |
| [43] | Khairiah, J., Ding-Woei, Y., Habibah, J., Ahmad-Mahir, A., Aminah, A. and Ismail, B. S. (2009). Concentration of heavy metals in guava plant parts and soil in the Sungai Wangi Plantation, Perak, Malaysia. International Journal of Agricultural Research 4: 310-316. |
| [44] | Ure, A. M. and Davidson, C. M. (2002). Chemical Speciation in the Environment. Blackwell, Oxford. |
| [45] | Rauret, G. (1998). Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 46: 449-455. |
| [46] | Amanda, J. Z and Weindorf, D. C. (2010). Heavy metal and trace metal analysis by sequential extraction: A review of procedures. International Journal of Analytical Chemistry 1-7. |
| [47] | Song, Q. J. and Greenway, G. M. (2004). A study of the elemental leachability of and retention capability of composts. Journal of Environmental Monitoring 6:31-37. |
| [48] | Ure, A. M., Davidson, C. M., Thomas, R. P. (1995). Single and sequential extraction schemes for trace metal speciation in soil and sediment. In: Quality assurance for environmental analysis. Elsevier Science B.V20: 505–523. |
| [49] | Fagbote, E. O. and Olanipekun, E. O. (2010). Evaluation of the status of heavy metal pollution of sediment of Agbabu bitumen deposit area, Nigeria. Eur. J. Sci. Res. 41(3): 373-382 |
| [50] | Charles, M. J. and Simmons, M. S. (1986). Methods for the determination of carbon in soils and sediments: A review. Analyst 111:385-390. |
| [51] | Broadbent, F. E. (1953). The soil organic fraction. Advances in Agronomy5: 153-183. |
| [52] | Nelson, D. W. and Sommers, L. E. (1996).Total carbon, organic carbon, and organic matter. In: Methods of Soil Analysis, Part 2, 2nd ed. Page, A. L., ed. American Society of Agronomy Inc. Madison, WI. 9: 961-1010. |
| [53] | Broadbent, F. E. (1953).The soil organic fraction.Advances in Agronomy5: 153-183. |
| [54] | Soil Survey Laboratory Methods Manual. (1992). Soil Survey Investigations Report No. 42. U.S. Department of Agriculture, Washington, DC. |
| [55] | Kholoud, M., Mohammed, A. and Yahya, A. (2009) Spatial Distribution and Environmental Implications of Lead and Zinc in Urban Soils and Street Dusts Samples in Al-HashimeyehMunicipalityJordan Journal of Mechanical and Industrial Engineering Jordan Journal of Mechanical and Industrial Engineering2:141 – 150. |
| [56] | Kabata-Pendias, A. (1995). Agricultural problems related to excessive trace metal contents of soil. In: Heavy metals (problems and solutions). Salomons, W., Förstnerand, U. and Mader, P. Eds. Springer Verlag, Berlin. 3-18. |
| [57] | Lindsay, W. L. (1979).Chemical equilibria in soils. John Wiley and Sons, New York. |
| [58] | Blom, H.A., 1986.Heavy metal contamination of soils around the cities of Ostfold County; Norway.Ph.D.Thesis, Norway Agricultural University, Norway, pp:102-113. |
| [59] | Yusuf, K.A. (2007).Sequential extraction of lead, copper, cadmium and zinc in soils near Ojota waste site.Journal of Agronom6:331– 337. |
| [60] | Surthland, R. A., Tolosa, C. A., Tack, F. M. G. and Verloo, M. G., (2000).Characterization of selected element concentration and enrichment ratios in background and anthropogenically impacted roadside areas.Archives of Environmental Contamination and Toxicology 38: 428–438. |
| [61] | Kashem, M.A, Singh, B.R., Kondo, T., ImamulHuq, S.M., Kawai, S. (2007). Comparison of extractability of Cd, Cu, Pb and Zn withsequentiel extraction in contaminated and non-contaminated soils. Int. J. Environ. Sci. Tech., 4(2), 169-176. |
| [62] | Sauve, S. (2001).Speciation of metals in soils. Bioavailability of metals in terrestrial ecosystems: Importance of partitioning for bioavailability to invertebrates. Microbes and Plants:7-38. |
| [63] | Sposito, G. (2008).The chemistry of soils 2nd edition: Oxford University Press. Madison Arenne, New York. |
| [64] | Kersten, M. and U. Förstner. 1986. "Chemical Fractionation of Heavy Metals in Anoxic Estuarine and Coastal Sediments.” Water Science and Technology 18: 121-130. |
| [65] | Kabala, C. and Singh, B. R. (2001). Fractionation and mobility of copper, lead, and zinc in soil profile in the vicinity of a copper smelter. Journal of Environmental Quality 30:485-495. |
| [66] | Olajire, A. A., Ayodele, E. T., Oyediran, G. O. and Oluyemi, E. A. (2003).Levels and speciation of heavy metals in soils of industrial southern Nigeria.Environmental Monitoring and Assessment 85:135-155. |
| [67] | Narwal, R. P., Singh, B. R. and Salbu, B. (1999).Association of cadmium, zinc, copper and nickel with components in naturally heavy metal rich soils studied by parallel and sequential extraction.Communications in Soil Science and Plant Analysis 30: 1209–1230. |
| [68] | Salbu, B., Krekling, T. and Oughton, D. H. (1998).Characterisation of radioactive particles in the environment.Analyst123: 843-849. |
| [69] | Karczewska, A. (1996). Metal species distribution on top and subsoil on an area affected by smelter emission. Applied Geochemistry 11: 35-42. |
| [70] | Sauve, S. (2003).The role of chemical speciation in bioavailability.Bioavailability, toxicity and risk.Relationships in Ecosystems:59-82. |
| [71] | Gupta, S. K and Chen, K. Y (1975). Partitioning of trace metal in selective chemical fractions of near shore sediments.Environmental Letters 10:129-158. |
| [72] | Madrid, L., Díaz-Barrientos, E. Madrid, F. (2002). Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere 49: 1301–1308. |
| [73] | Tukura, B W., Kagbu, J A. and Gimba, C. E. (2007). Effects of pH and total organic carbon on the distribution of trace metals in Kubanni dam sediments, Zaria, Nigeria. Science World Journal 2:1-6. |
| [74] | Iwegbue, C. M. A., Nwajei, G. E., Eguavon, O. and Ogala, J.E. (2009). Chemical fractionation of some heavy metals in soil profiles in vicinity of scrap dumps in Warri, Nigeria. Chemical Speciation and Bioavailability 21: 99-110. |
| [75] | Oviasogie, P. O. and Ofomaja, A. (2007). Available Mn, Zn, Fe, Pb and physicochemical changes associated with soil receiving cassava mull effluent. Journal of Chemical Society of Nigeria 32:69-73. |
| [76] | Isirimah, N. O. (1987). An inventory of some chemical properties of selected surface soils of River state of Nigeria: In proceeding of the 15th annual conference of soil science association of Nigeria, Kaduna. 217-233. |
| [77] | Odu, C. T. I., Esurosu, O. F., Nwaboshi, I. C. and Ogunwale, J.A. (1985).Environmental study (Soil and Vegetation) of Nigeria Agip oil company operation area. A report submitted to Nigeria Agip Oil Company Limited, Lagos, Nigeria. 102-107. |
| [78] | Sauvé, S., Hendershot, W. and Allen, H. E. (2000). Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environmental Science and Technology34: 1125-1131. |
| [79] | Munoz-Melendez, G., Korre, A. and Parry, S. J. (2000). Influence of soil pH on the fractionation of Cr, Cu and Zn in solid phases from a landfill site. Environmental Pollution 110:497–504. |
| [80] | Zhang, T., Shan, X. and Fuliang, L. (1998).Comparison of two sequential extraction procedures for speciation analysis of metals in soils and plant availability.Communication in Soil Science and Plant Analysis 29: 1023-1034. |
| [81] | Zhang, H. and Young, S. D. (2005).Characterizing the availability of metals in contaminated soils.The soil solution Soil Use and Management 21:459-467. |
| [82] | Smolders, E., Oorts, K., van-Sprang, P., Schoeters, I., Janssen, C. R., McGrath, S. P. and McLaughlin, M. J. (2009). Toxicity of trace metals in soil as affected by soil type and aging after contamination: Using calibrated bioavailability models to set ecological soil standards. Environmental Toxicology and Chemistry 28:1633-1642. |
| [83] | Sheppard, S. C., Evenden, W. G. and Schwartz, W. J. (1995). Heavy metals in the environment, ingested soil: bioavailability of sorbed lead, cadmium, cesium, iodine, and mercury. Journal of Environmental Quality 24: 498-505. |
| [84] | United Nations Environmental Protection Programme (UNEP), Nairobi. (2004). Sustainable consumption and production activities in Africa: Regional status report, 2002-2004. Nairobi Kenya. |
| [85] | Ayodele, J. T. and Oluyomi, C. D. (2011).Grass contamination by trace metals from road traffic. Journal of Environmental Chemistry and Ecotoxicology 3:60-67. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML