-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2013; 3(3): 102-107
doi:10.5923/j.env.20130303.05
Sharp Decline in Lead Contamination in Topsoil Away From a Smelter and Lead Migration in Ultisol
Sigurdur Greipsson1, Charlotte Tay2, Alicia Whatley2, Daniel M. Deocampo3
1Biology and Physics Department, Kennesaw State University, 1000 Chastain Rd., Kennesaw, GA 30144, USA
2Dept. of Biological and Environmental Sciences, Troy University, Troy, Alabama, USA
3Dept. of Geosciences, Georgia State University, Atlanta, Georgia, USA
Correspondence to: Sigurdur Greipsson, Biology and Physics Department, Kennesaw State University, 1000 Chastain Rd., Kennesaw, GA 30144, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The spatial distribution of lead (Pb) contamination in soil was examined in Pike County, Alabama. Soil samples were collected from 16 sites away (0.2 to 18.44 km) from a smelter. Distribution of Pb into the soil profile was also examined in three sites close (within 3.0 km) to the smelter. Results showed that Pb contents (max. 199.8 mg kg-1) in the topsoil were strongly influenced by the distance from the smelter; with marked increase in values of Pb contents when the distance was less than 3.0 km. Soil physical and chemical properties did not influence the spatial distribution of topsoil Pb contents. Results revealed that in close vicinity (0.2 km) to the smelter Pb accumulates in the topsoil and in the E and Bt horizons. This site had low soil pH (3.7) that may have contributed to the distribution of Pb in the E and Bt horizons. Controlled soil column experiment demonstrated Pb mobility only in soil close (0.2 km) to the smelter. Lead concentration in the soil leachate was 71 µg L-1 for the soil close to the smelter but was not detected in the leachate of the soil away (3.4 km) from the smelter. This suggests that Pb in soil close to the smelter has potential to migrate through the soil profile.
Keywords: Alabama, Pb, Pb-migration, Smelter, Ultisol
Cite this paper: Sigurdur Greipsson, Charlotte Tay, Alicia Whatley, Daniel M. Deocampo, Sharp Decline in Lead Contamination in Topsoil Away From a Smelter and Lead Migration in Ultisol, World Environment, Vol. 3 No. 3, 2013, pp. 102-107. doi: 10.5923/j.env.20130303.05.
Article Outline
1. Introduction
- Atmospheric deposition of lead (Pb) including leaded gasoline and Pb-smelters has been associated with widespread Pb contamination in soils in USA[1, 2, 3, 4]. Soils surrounding Pb-smelters are particularly prone to high levels of Pb contamination[5]. Soil contamination by Pb may have dire consequences such as loss of agricultural productivity, diminished environmental quality and contaminated water resources[4]. Lead has no known biological role and there is a public concern about health effects resulting from Pb pollution[6]. Topsoil is a major receptor of atmospheric Pb pollution[1]. Whereas road dust rich in Pb may be easily transported by storm water into streams, Pb is quite persistent once deposited in soils[7, 8]. The geochemical behavior of Pb in soils such as downward movement through the soil profile is influenced by soil physical and chemical properties[9]. Soil pH affects the mobility of heavy metals in soil by influencing the rate of solubility and adsorption to colloids[10, 11]. Lead is particularly soluble and mobile in acidic soil[10, 11, 12]. Lead is typically persistent in organic layers of soils where it forms strong complexes with soil organic carbon[13, 14, 15]. The distribution of metal contaminants, with progressive depth in soil profiles, can indicate downward movement of metals that originate from surface deposition[16, 17, 18, 19]. In addition, migration of metals through soil profile can be estimated under controlled condition by soil column studies[20].The overall spatial distribution of heavy metal contamination, together with possible downward movement of heavy metals through the soil profile provides crucial information prior to remediation of contaminated soils[21, 22]. Soil remediation could involve phytoextraction[23, 24]. In order to assess the extent of the environmental impact of the activities of the smelter in Troy the spatial distribution of Pb in topsoil content at 16 sites in Pike County, Alabama was studied. In addition, distribution of Pb in different soil horizon was examined in three sites close to the smelter to obtain information on potential mobility of Pb through the soil profile. Also, migration of Pb was estimated under controlled conditions in a soil column study. Soil physical and chemical properties were given special attention to emphasize their significance in the evaluation of the geochemical behavior of Pb in contaminated soils.
2. Materials and Methods
2.1. Study Area
- The smelter in Troy (latitude 31.78627106º: longitude 85.97862228º) mainly recycles Pb-acid batteries into refined Pb alloys and has been operating since 1970. Emission data from the EPA’s National Emission Inventory (NEI) database for 2002 showed that the smelter in Troy emitted 4.43 tons of Pb per year (tby)[25]. The study area containing the 16 sites covered radial distances of approximately 18.44 km north (N), 10.75 km south (S), 9.75 km northwest (NW), and 18.4 km northeast (NE) from the smelter (Fig. 1a). The study area included industrial site, urban, suburban and rural area.
2.2. Soil
- Ultisols are the dominating soil type of much of the southeastern USA. Ultisols are highly weathered soils with distinctive horizons, strongly leached and have relatively low fertility. Ultisols are typically acidic and average pH of soils in Pike County was about 5.5[26]. Soil samples were collected using an Eldermeyer auger from the A horizon (max 10 cm depth). Three replicated soil samples were collected from each site and each replicate was taken 3 m apart. Secondly, the distribution of Pb in the soil profile was examined in three sites. These sites were close to the Smelter; Site 1 (600 m), Site 2 (1560 m) and, Site 3 (2840 m). At each site samples were collected from three soil pits that were 3 m apart. At each soil pit five samples were collected from three soil horizon (A, E and Bt). The soil type of these three sites was Lucy loamy well drained sand with a depth of about 1.5 m[26]. The soil samples were stored in Ziploc bags, kept in a cooler in the field to prevent fermentation and exhaustion of carbon by microbial activity, and then transferred to a refrigerator at 4℃ for storage prior to analysis.
2.3. Methodology
- The soil grain size was estimated by using standardized soil sieves to separate the sand (0.05 to 2.0 mm), from the silt and clay particles. The combined weight of silt and clay was placed in a beaker, water was added and mixed, and silt was allowed to settle for 15 minutes to the bottom of the beaker while the clay was retained in suspension. The clay/water solution was poured off and the silt was dried in an oven at 55℃ overnight. The dried silt was then weighed. Soil samples were dried in an oven at 55℃ to remove water prior to determining the amount of soil organic carbon in the soil. Soil organic carbon was estimated by the loss-on-ignition (LOI) method by placing a known weight of dried soil in a crucible that was placed in a muffler furnace (450℃) for 24 hours. The LOI is commonly used to estimate soil organic carbon in soils with low clay content[27]. The soil pH was determined using a pH meter (Orion model 290A plus). The pH measurements were taken 30 minutes after mixing 20 g of air-dried soil with 20 mL of deionized water[28]. The cation exchange capacity (CEC) was estimated according to[29].The soil extraction process was a modification of the procedure used by Soon and About[30]. Soil samples were oven-dried for 12 hr at 55℃. Dry subsamples (4 g) were digested with 25 ml of concentrated HNO3 (nitric acid) prior to extraction. Samples were stirred for 2 hr at room temperature. Samples were heated (90℃) to dryness. The soil extraction solution (50 ml) was made up of 0.1 M HNO3 and 0.05 M Na4-EDTA (ethylene diamine tetraacetic acid). The soil extraction solution was heated (90℃) and stirred for 30 min. The extraction solution was filtered (Whatman no. 11) using a vacuum pump. The filtered solutions were analyzed for Pb using a flame atomic absorption spectrometer (AAS) (Perkin Elmer AAS model 3100; detection limit = 0.5 ppm) at the Chemistry Department of Troy University, Troy, Alabama. To determine the recovery rate of the extraction procedure, soil samples (n=5) were spiked with known contents of Pb, after which Pb was extracted from the soil. The percentage recoveries were between 88-95% (75% of the samples ≥ 91% recovery). In order to check for drift in the AAS used in the determination of Pb, standard solutions of known Pb contents and blank samples were analyzed for every 5 samples. The AAS was calibrated with Pb standard solutions prepared from Pb atomic absorption standard solution (Sigma Aldrich Chem. Company, Inc.) prior to Pb analysis. The extraction solutions from the soil samples taken at different soil horizons were analyzed using the inductively coupled plasma mass spectrophotometer (ICP-MS; detection limit = 0.5 ppm) at the Soil Testing Laboratory, Auburn University, Auburn, AL.Glass columns (2 cm diameter) were gently packed with 100 ml of soil. Topsoils form two sites were tested in triplicated columns. The first soil was from the site closest (0.2 km) to the smelter and the second one was from a site 3.4 km away from the smelter. The Pb concentration of the topsoil close to the smelter was 199.8 mg kg-1 and 9.3 mg kg-1 in the soil of the site further away from the smelter. A 425 µm mesh (Monodur®) was placed at the bottom of the column. Columns were adjusted vertically and gently shaken during soil packing. Soil was prewetted in the columns by pouring deionized water slowly to the top of the soil. The soil leaching was conducted by adding deionized water slowly to the top of the soil until 25 ml of leachate was collected in the sampling bottle. The leachates were filtered through 2 µm Teflon filter (Environmental Express Inc.) and analyzed the same day for total Pb at the Geoscience Dept. of Georgia State University using mass atomic absorption spectrometer (Perkin Elmer model 3100). Statistical analyses were performed using Excel Microsoft statistical software package. Both linear and non-linear regression analyses were performed to determine the correlation between Pb content and distance from the smelter. Significant relationships between Pb content and soil physical and chemical properties were determined using linear regression models.
3. Results and Discussion
- Non-linear regression analysis showed a very strong inverse asymptomatic relationship between Pb content and distance from the smelter with dramatic increase in values of Pb content when a distance was less than 3.0 km (Fig. 1b, 2). Distance from the smelter accounted for about 85% of the variation in Pb contents (r2 = 0.85, p < 0.05). This indicates strongly that distance from the smelter was a major factor affecting topsoil Pb content.
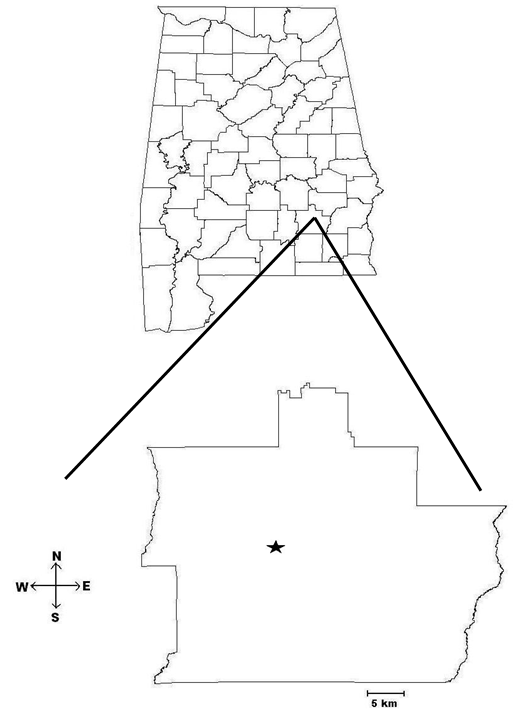 | Figure 1a. Map of Alabama, USA and Pike County. The city of Troy is indicated with a star |
 | Figure 1b. Map of Pike County showing topsoil Pb concentrations (mg kg-1) recorded at the different sampling sites |
 | Figure 2. Relationship between soil Pb contents and distance from the smelter. Best fit regression for soil Pb content was y = 44.567x-0.81, r2=0.85 |
|
- These findings are consistent with previous studies that have shown Pb contamination in topsoils to decrease from the contaminating source[28, 31]. In previous studies involving lichens as bio-indicators of Pb pollution, sampling sites downwind of the smelter recorded high levels (>2000 mg kg-1) of Pb in lichens[32]. In this study, soil Pb contamination from smelting activities was highest for soil samples collected within 3.0 km from the smelter (Figure 1). The highest mean value of soil Pb (199.8 mg kg-1) was recorded from the site that was closest (0.2 km) to the smelter. Our results agree with previous studies[28, 33], that the extent of topsoil contamination with Pb from the smelter in Pike County, AL is localized and the level of Pb-contamination is generally low. The Pb content of soils in the Atlantic Coastal Plain of USA is generally low, with concentrations equal to or less than 14 mg kg-1[9]. In Florida, mean Pb content for Ultisol topsoil was 12.1 mg kg-1[34].The relationship between soil physical and chemical properties and the contents of Pb in contaminated topsoils at different sampling sites were none. The topsoil was generally very strongly acidic (mean pH 4.7) and the lowest value of a single sample was ultra acidic (pH 2.9) derived from one of the samples on the site located 0.6 km to the smelter[35]. Another study found the pH of the topsoils to be extremely acidic to moderately acidic (3.8 to 5.7)[26]. No relationship was found between soil Pb contents and cation exchange capacity (CEC) of soils. Surface deposited Pb has previously been reported to accumulate within topsoils[17, 36]. In this study Pb accumulated in the topsoil close (within 3.0 km) to the smelter. The distribution of Pb observed in different soil horizons of three sites close to the smelter is shown in Table 1. Enrichment in Pb contents in the A, E and Bt horizons was demonstrated in site 1 strongly suggesting a downward movement of Pb (Table 1). The Pb values were though sharply diminished from A horizon (99.7 mg kg-1) to Bt horizon (18 mg kg-1). On site 2 the A and E horizon showed enriched Pb contents. The exceptionally low Pb content in the Bt horizon on site 2 is possibly the result of metal leaching due to very acidic soil (Table 1). In the soil column experiment Pb was only found in the leachate of the soil close to the smelter (Table 2). The Pb concentration in this soil leachate was 71±4 µg L-1 (Table 2). This strongly suggests that Pb has a great potential to migrate through the soil profile on the site close to the smelter. The dynamic process of Pb downward movement through the soil profile on the site close to the smelter must be studied further especially considering that this process has taken place in 40 years. It is critical to consider soil pH to understand metal geochemical behavior in soil systems[14]. Generally, desorption of metals is increased as pH decreases; hence metals tend to be soluble in more acidic soils[37]. Other studies have reported much higher values of Pb-contamination of soils adjacent to Pb-smelters. For example in Missouri Pb levels of soils were in excess of 60,000 mg kg-1[38]. The results demonstrated that Pb accumulates in soils close to the smelter. However, the extremely acidic soil close to the smelter most likely contributed to the distribution of Pb into the soil profile. Also, the soil column experiment demonstrated Pb mobility of soil close to the smelter. The topsoils from the two sites closest to the smelter were extremely acidic (pH 3.7 and 3.9) and most likely the low pH played a role in the distribution of Pb within the soil profile (Table 1) and high Pb concentration in the soil leachate of soils close to the smelter. The pH values of soil in the E and Bt horizons at these two sites were also extremely acidic (4.0 and 4.2) (Table 1). The downward movement of Pb through the soil profile is enhanced in acidic soils compared to neutral or alkaline soils. It was previously observed that most of heavy metals (Pb included) remained in the upper layers of loamy soils at neutral pH, but at decreased pH of 5.7, dissolution of metals and movement to lower depths in the soil profile was observed[17].
4. Conclusions
- This study revealed that the extent of Pb-contamination (max. 199.8 mg kg-1) of top soil in Pike County, Alabama is localized in close vicinity (within 3.0 km) of an active smelter. The top soil Pb contamination was reduced drastically over a short distance (3.0 km) away from the smelter. The relationship between soil physical and chemical properties and the contents of Pb in contaminated top soils at different sampling sites were none. The results demonstrated that Pb accumulates in soils close to the smelter. However, the extremely acidic soil close to the smelter most likely contributed to the distribution of Pb into the soil profile. Determining the spatial distribution of Pb content in the top soil in Pike County along with information on potential downward movement of Pb through the soil profile will assist in identifying contaminated areas that may require future remediation.
References
| [1] | Adriano DC (1986) Trace elements in the terrestrial environment. Springer-Verlag, New York |
| [2] | Ripley EA, Redmann RE, Crowder AA, Ariano TC, Farmer RJ (1996) Environmental Effects of Mining. St. Lucie Press, Delray Beach |
| [3] | Kabata-Pendias A, Pendias H (1992) Trace element concentrations in soils and plants (2nd ed). CRC Press, Boca Raton |
| [4] | Forstner U (1995) Land contamination by metals- Global scope and magnitude of problem. In: Bowers AR, Allen HE, Huang CP, Bailey GW (eds) Metal speciation and contamination of soil. CRC Press, Boca Raton |
| [5] | Mbila MO, Thomson LM (2004) Plant-available zinc and lead in mine spoils and soils at the mines of Spain, Iowa. Journal of Environmental Quality 33:553-558 |
| [6] | Lanphear BP, Hornung R, Khoury J, Yoltonm K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894-9 |
| [7] | Filippelli GM, Laidlaw M, Raftis R, Latimer JC (2005) Urban lead poisoning and medical geology: An unfinished story. GSA Today, 15:4-11 (doi: 10.1130/1052- 5173(2005)015 < 4:ULPAMG>2.0.CO;2) |
| [8] | Deocampo DM, Reed PJ. Kalenuik AP (2012) Road Dust Lead (Pb) in Two Neighborhoods of Urban Atlanta (GA, USA). International Journal of Environmental Research and Public Health 9:2020-2030 |
| [9] | Howard JL, Sledzinski G (1996) Geochemical Behavior of Lead in an Alfisol and an Ultisol at High Levels of Contamination. Soil and Sediment Contamination 5:61-81 |
| [10] | Reddy KJ, Wan L, Gloss SP (1995) Solubility and mobility of copper, zinc and lead in acidic environments. Plant and Soil 171:53-58 |
| [11] | Brady NC, Weil RR (2002) The nature and properties of soils. Prentice Hall, New Jersey |
| [12] | Lee SS, Chang L, Yang HH, Chen CM, Liu MC (1998) Adsorption characteristics of lead onto soils. Journal of Hazardous Materials 63:37-49 |
| [13] | Sheppard MI, Thibault DH (1992) Desorption and extraction of selected heavy metals from soil. Soil Sci Soc Am J 56:415-423 |
| [14] | McBride MB (1994) Environmental chemistry in soils. Oxford University Press, Oxford |
| [15] | McGrath SP, Zhao FJ, Lombi E (2001) Plant and rhizosphere processes involved in phytoremediation ofmetal-contaminated soils. Plant and Soil 232:207-214 |
| [16] | Sipos P, Nemeth T, Mohai I (2005) Distribution and possible immobilization of lead in the forest soil (Luvisol) profile. Environ. Geochem. Health 27:1-10 |
| [17] | Scocart PO, Meeus-Verdinne K, DeBorger R (1983) Mobility of heavy metals in polluted soils near zinc smelters. Water, Air and Soil Pollution 20:451-463 |
| [18] | McGowen SL, Basta NT (2001) Heavy metal solubility and transport in soil contaminated by mining and smelting. In: Selim HM & Sparks DL (eds) Heavy metal release in soils. CRC Press, Boca Raton |
| [19] | Udom BE, Mbagwu JSC, Adesodun JK, Agbim NN (2004) Distributions of zinc, copper, cadmium and lead in a tropical ultisol after long-term disposal of sewage sludge. Environment International 30:467-470 |
| [20] | Camobreco, VJ, Richards BK, Steenhuis TS, Peverly JH, McBride MB (1996) Movement of heavy metals through undisturbed and homogenized soil columns. Soil Science 161:740-750 |
| [21] | Sánchez-Martin MJ, Lorenzo LF, Sánchez-Camazano M (2001) Leaching of Cd, Zn, Pb, and Cu in packed and undisturbed columns of soils affected by the spill from a pyrite mine in the south of Spain. Soil and Sediment Contamination 10:359-373 |
| [22] | Williford CW, Bricka RM (2001) Physical separation of metal-contaminated soils. In: Iskandar IK (ed) Environmental restoration of metals-contaminated soils. CRC Press, Boca Raton |
| [23] | Greipsson S (2011) Phytoremediation. Nature Education 3(10):7 |
| [24] | Perry VR, Krogstad EJ, El-Mayas H, Greipsson S (2012) Chemically Enhanced Phytoextraction of Lead-Contaminated Soils. International Journal of Phytoremediation 14:703-713 |
| [25] | www.epa.gov/air/emissions/pb.htm#pbloc |
| [26] | USDA, Natural Resources Conservation Service (1997) Soil Survey of Pike County, Alabama. National Cooperative Soil Survey |
| [27] | Howard PJA, Howard DM (1990) Use of organic carbon and loss-on-ignition to estimate soil organic matter in different soil types and horizons. Biology and Fertility of Soils 9:306-310 |
| [28] | Anderson S, Chappelka AH, Flynn KM, Odom W (2000) Lead accumulation in Quercus nigra and Q. velutina near smelting facilities in Alabama, U.S.A. Water, Air and Soil Pollution 118:1-11 |
| [29] | Rhoades JD (1982) Cation exchange capacity: Methods of soil analysis. Part 2. In: Page AL, Miller RH, Keeney DR (eds) Chemical and microbiological properties Agronomy Series No. Part 2. American Society of Agronomy, Madison |
| [30] | Soon YK, Abboud S (1993) Cadmium, chromium, lead, and nickel. In: Carter MR (ed) Soil Sampling and Methods of Analysis. Lewis Publishers, Boca Raton |
| [31] | Massadeh, AM, Tahat M, Jaradat QM, Al-Momani IF (2004) Lead and cadmium contamination in roadside soils in Irbid city, Jordan: A case study. Soil and Sediment Contamination 13:347-359 |
| [32] | Gibson SP, Morris CC, Hovsepyan A, Greipsson S (2002) Atmospheric deposition of lead in Troy, Alabama using lichens as bioindicators. Southeastern Biology 49:209-210 |
| [33] | Judah LS (2004) Heavy metal contamination of soils at five polluted sites in southeast Alabama. MS Thesis. Troy University, Troy, AL |
| [34] | Ma QL, Tan F, Willis WG (1997) Concentrations and distributions of eleven metals in Florida soils. Journal of Environmental Quality 26:769-775 |
| [35] | Soil Survey Division Staff (1993) Soil Survey Manual. USDA Handbook 18,: U.S. Government Printing Office, Washington |
| [36] | Smith, W.H. (1990). Air Pollution and Forests (2nd ed.). Springer-Verlag, New York |
| [37] | Impellitteri CA, Allen HE, Yin Y, You Z, Saxe JK (2001) Soil properties controlling metal partitioning. In: Selim HM, Sparks DL, (eds) Heavy metal release in soils. CRC Press, Boca Raton |
| [38] | Palmer KT, Kucera CL (1980) Lead contamination of sycamore and soil from lead mining and smelting operations in eastern Missouri. J Environ Qual 9:109-111 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
