-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2013; 3(2): 60-65
doi:10.5923/j.env.20130302.04
Mammalian Feces as Bioindicator of Urban Air Pollution in Captive Mammals of Jaipur Zoo
1Mahatma Gandhi Institute of Applied Sciences, JECRC Foundation, Jaipur, 302022, India
2JECRC University, Sitapura Industrial area, Jaipur, 302022, India
Correspondence to: Prakash Bakre, JECRC University, Sitapura Industrial area, Jaipur, 302022, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
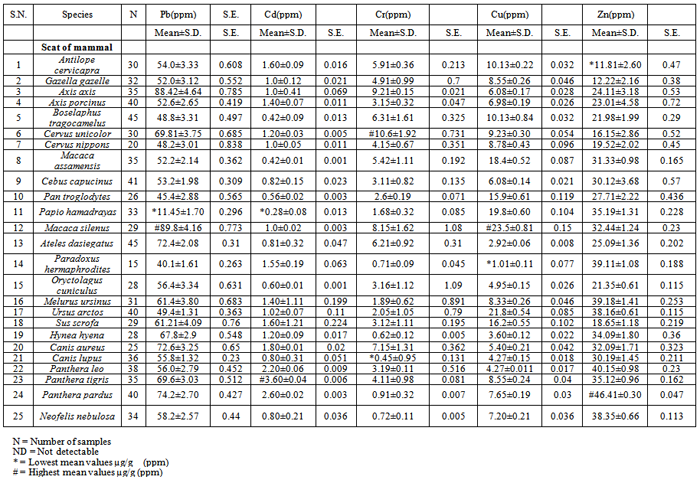
Study of environmental contamination in the wildlife, free-ranging or those caged in the zoos, is a challenging venture. This is primarily because of difficulty in obtaining samples, which can be at the most opportunistic, from these animals. Feces of wild animals were used as biological indicator of exposure in wild animals caged and exposed to the ambient air pollution of urban habitat. Various metal contents in mammals of Jaipur (India) zoo were in the range of 89.8±4.16 (Macaca silenus) to 11.45±1.70 (Papio hamadrayas) ppm d/w in case of lead. Cadmium was in range between 3.60±0.04 (tiger, Panthera tigiris) to 0.28±0.08 (hamadrayas baboon, Papio hamadrayas) ppm d/w. Chromium was in rage of 10.6±1.92 (Cervus unicolor) to 0.45±0.95 (Canis lupus) ppm d/w. Copper was in range between 23.5±0.81(Macaca silenus) to 1.01±0.11(Paradoxus hermaphroditus) ppm d/w. Whereas zinc was found in range of 46.41±0.30 (Panthera pardus) to 11.81±2.60 (Antilope cervicapra) ppm d/w. Analysis of feed and water along with the soil in cages which is receiving particulate air pollutants indicates that air pollution is the primary cause due to high density of traffic in the area.
Keywords: Air Pollution, Heavy Metals, Bioindicator, Feces, Wild Mammals
Cite this paper: Varsha Gupta, Prakash Bakre, Mammalian Feces as Bioindicator of Urban Air Pollution in Captive Mammals of Jaipur Zoo, World Environment, Vol. 3 No. 2, 2013, pp. 60-65. doi: 10.5923/j.env.20130302.04.
1. Introduction
- Human health is a primary concern in most of the air monitoring studies in urban environment. A group of animals housed in zoo, and sharing the urban air, are least studied. Most of the Indian zoos were located away from the cities to begin with. However, all the Indian cities have outgrown well beyond their original limits. As a result majority of the zoos are now surrounded by human habitations. Jaipur zoo was established in 1887 and was located on the outskirts of the walled city. Most of the new colonies have sprung up beyond the zoo. A road that bisects the zoo connects the new localities and the old walled city. Everyday an average of more than lakhs of vehicles pass on this road. Most of these vehicles are petrol driven and include cars, motorcycles, scooters, and mopeds. The cages of animals are too close to this road. The nearest cages harbouring major carnivora, like Lion (Panthera leo), Tiger (Panthera tigris) and Leopard (Panthera pardus), which are barely 25 meters away from this road. The automobiles ply on this road continuously emitting exhaust and since the animals are confined to cages they are continuously exposed to the automobile exhaust.Ingestion of Pb containing paint from bars and walls have been reported to be a significant cause of death among captive wild animals including monkeys, bears, raccoons, armadillos etc.[1],[2]. Similar situation was reported for domestic animals like dogs, cats, goats, cattle etc.[3]. The highest body burdens of Pb were reported in mammals near urban areas with dense vehicular traffic and also near metal mines and smelters[4].Signs of lead toxicosis in cows (Bos bovins) included muscle tremors, blindness, dribbling urine and drooling. Proximity to smokestacks of metal smelters was positively associated with increased level of lead in hair (manes) of horses and in tissues of small mammals which was consistent with the results of soil and vegetable analysis.Several studies have reported concentrations of metals in wild mammals living in highly contaminated area near smelters[5], chlor-alkali plant[6],[7], verges of heavily- used highways[8] and mines or mine waste sites[9-10],[11].Various methods were employed to assess and draw a concentration profile of a variety of pollutants that might reach the wildlife habitats and wildlife itself. In fact the human race in its selfish design has used wildlife species as biological indicators to study the ambient concentration of the toxicants in his own ecosystem, both urban and industrial. However, mammals, which are much closer to human beings, are rarely used. Rats, captured from either side of the highways indicated that the body concentration of the lead was directly proportional to the distance from the highway [12].Bat was the first mammal used for analysis of its guano as bio-indicator for pesticidal pollution as well as mercury exposure[13],[14],[15] and analysis of feces for Cd intake in humans[16]. Concentration of cadmium, lead, zinc, copper were studied in the feces of deer killed near smelters to check the degree of metals pollution[17]. Fecal level of metals were four fold higher in urban than the rural rats[18].A pilot study to monitor Pb contamination in wild herbivores from the protected areas of Rajasthan, India[19] suggests that exposure to heavy metals can be studied using herbivore dung as a bio-indicator. In the continuation of this, study was also done in mammalian fauna of Keoladeo National Park, Bharatpur[20]. Scat samples of the mammals, vegetation, and soil samples clearly indicate the extent to which the mammalian fauna is exposed to metal contamination.However, the method of sacrificing or killing of animal may appear more scientific, but is certainly ethically unsound. Given the concern for loss of animal lives for scientific investigation, and the increasing biological poverty of the planet earth, there is an urgent need for developing biological indicator which will not involve killing of animals. To overcome this problem it was proposed to use feces / scat / fecal matter as bio-indicators or as a biomarkers to study exposure to heavy metals.
2. Material and Methods
- Fresh scat samples of mammals housed in the animal section of Jaipur zoo, India, were collected from the cages with the help of zoo staff. Samples were brought to the laboratory and freeze dried. Scat samples were collected from the cases of following mammalian species; sambar (Cervus unicolor), chital (Axis axis), hog deer (Axis porcinus), sikka deer (Cervus nippons), nilgai (Boselaphus tragocamelus), chinkara (Gazella gazella), blackbuck (Antilope cervicapra), spider monkey (Ateles dasiegatus), capuchin monkey (Cebus capucinus), lion-tailed macaque (Macaca silenus), chimpanzee (Pan troglodytes), hamadrayas baboon (Papio hamadrayas), assamees macaque(macaca assamensis), sloth bear (Melurus ursinus), brown bear (Ursus arctos), rabbit (Oryctolagus cuniculus), wild boar(Sus scrofa), civet (Paradoxus hermaphroditus), Asiatic lion (Panthera leo persica), leopard (Panthera pardus), tiger (Panthera tigris), clouded leopard (Neofelis nebulosa), jackal (Canis aureus), striped hyena (Hynea hynea), and wolf (Canis lupus). To ascertain the source of contamination water and food samples of this zoo were also collected. Another, suspected source of contamination was suspended particulate matter settling on the floor of cages, hence soil samples were also taken from cages of animals. Scat and soil samples were stored in the plastic ziplock bags and water samples in the sterilized plastic containers.For analysis of sample 0.5 gm of dry scat / vegetation / feed / soil were weighed and taken in the hard Borosil glass tube. Concentrated nitric acid and perchloric acid were added to each sample in 4:1 ratio. Sample was kept in water bath for 5 to 6 hours or until it was digested completely and became clear. When the sample was clear 3 to 4 drops of H2O2
 were added to neutralize and to dissolve the fat. After cooling each sample was diluted upto 10 ml with deionized water and transferred to sterilized Borosil glass vial and stored at room temperature prior to analysis.Water samples were transferred into beakers, cleaned with double distilled and acidified distilled water, and concentrated keeping on a hot plate in a flame hood adding 12 to 15 ml of analytical grade HNO3. The heating was continued till such time the sample became colorless and clean. However, samples were never allowed to dry completely. By and large, nitric acid alone was adequate for complete digestion of water samples. HClO4 was added only to those samples which had high organic matter which were always treated in advance (pre-treated) with nitric acid before adding perchloric acid. If necessary, more HNO3 was added and volume brought down to the lowest quantity (10 to 25 ml) before precipitation occurred. After completing the digestion, beakers were allowed to cool. Samples were diluted upto 10 ml with double distilled water. Entire metal analysis was done by using GBC Advanta ver. 1.31 Atomic Absorption Spectrophotometer at 217 nm for lead, 228.9 nm for cadmium, 324.7 nm for copper, 213.9 nm for zinc and 357.9 nm for chromium. Results are presented in µg/g (ppm) dry weight and µg/ml (ppm) wet weight.
were added to neutralize and to dissolve the fat. After cooling each sample was diluted upto 10 ml with deionized water and transferred to sterilized Borosil glass vial and stored at room temperature prior to analysis.Water samples were transferred into beakers, cleaned with double distilled and acidified distilled water, and concentrated keeping on a hot plate in a flame hood adding 12 to 15 ml of analytical grade HNO3. The heating was continued till such time the sample became colorless and clean. However, samples were never allowed to dry completely. By and large, nitric acid alone was adequate for complete digestion of water samples. HClO4 was added only to those samples which had high organic matter which were always treated in advance (pre-treated) with nitric acid before adding perchloric acid. If necessary, more HNO3 was added and volume brought down to the lowest quantity (10 to 25 ml) before precipitation occurred. After completing the digestion, beakers were allowed to cool. Samples were diluted upto 10 ml with double distilled water. Entire metal analysis was done by using GBC Advanta ver. 1.31 Atomic Absorption Spectrophotometer at 217 nm for lead, 228.9 nm for cadmium, 324.7 nm for copper, 213.9 nm for zinc and 357.9 nm for chromium. Results are presented in µg/g (ppm) dry weight and µg/ml (ppm) wet weight.3. Results and Discussion
- Concentration of lead, cadmium, chromium, copper and zinc in scat / fecal matter was analysed for every mammalian species captivated in a similar environment of zoo. These results show a trend of variation in metal content according to the feeding habits as well as activity level of mammals. The mammals were categorized in three major groups i.e. herbivores that feed on green leaves (vegetation), vegetables, green grains, fruits, cereals, pulses etc., omnivores which feed on both vegetation and meat or fish and carnivores type which are fed meat and fish. Metals concentrations indicate gross exposure.The concentration of lead analyzed in fecal matter of captive zoo wild mammals was in the range of 89.8±4.16 (lion-tailed macaque) to 11.45±1.70 ppm d/w. Cadmium was in range between 3.60±0.04 (tiger) to 0.28±0.08 (hamadrayas baboon) ppm d/w. Chromium was in rage of 10.6±1.92 (sambar) to 0.45±0.95 (wolf) ppm d/w. Copper was in range between 23.5±0.81 (lion-tailed macaque) to 1.01±0.11 (civet). Whereas zinc was found in range of 46.41±0.30 (panther) to 11.81±2.60 (blackbuck) ppm d/w (Table 1).The background levels of lead, cadmium, chromium, copper and zinc in food were analysed. The feed of every mammalian species was analyzed and it was found that lead was present in each sample of food which was provided to zoo mammals (Table 2). The concentration of lead was found in the range of 0 to 29.8 ppm d/w. Cadmium was found in range of 0 to 1.01 ppm d/w. The concentration of chromium was found in the range of 0.31 to 15.32 ppm d/w. Copper was analysed in the range of 10.84 to 39.7 ppm d/w. The concentration of zinc in feed samples was observed in the range of 0 to 46.4 ppm d/w.The background level of lead, cadmium, chromium, copper and zinc in soil and water were also analysed. The concentration of lead in soil was found to be significantly high 10.6±2.29 ppm d/w. Water was found to have trace amount of lead contents i.e. 0.24 ± 0.24 ppm w/w. Cadmium concentration in soil and water were significantly lower i.e. 0.60±0.85 ppm d/w and 0.12 ± 0.02 ppm w/w. Chromium concentration in soil and water were. 15.32±1.29 ppm d/w and 1.81±0.60 ppm w/w. Copper concentration of soil and water were found to be 16.6±0.15 ppm d/w and 3.9±0.08 ppm w/w. In case of soil and water zinc content was 27.8±2.01 ppm d/w and 10.24±0.05 ppm w/w respectively.Lead, cadmium, chromium, copper and zinc concentration were found in considerable amount in the biological samples (fecal matter/ feed) and non-biological (soil/water) samples collected from Jaipur zoo. Concentration of metals in particularly in fecal matter samples from zoo is much higher than the wild animals like white tailed deer feeding near smelter[17]. Study of Jaipur zoo shows that a part of exposure of mammals is through food while the metals in water were in traces. Leonzio and Massi [21] had shown that metal concentration in feces normally equals that in food. Obviously the additional exposure was through plausible route of inhalation. The load of lead in fecal matter almost exceeded what is present in the food material.Jaipur zoo apparently is with most polluted one for the obvious reason that it is situated on a road which connects the walled city with new colonies and more than lakhs of vehicles pass on this road in a day. Wild mammals housed in zoo have no choice but to inhale the automobile exhaust, being caged, all 24 hours. Fortunately, NEERI, India[22] had conducted air monitoring studies showing the presence of metals in the urban air of Jaipur city which appears to be the possible route of exposure and presence of metals indicates the gross exposure of wild mammals cages in zoos in immediate past 24 hours. Soils receive potentially toxic elements from both natural and wide range of anthropogenic sources, including the weathering of primary minerals, mining, fossil fuel combustion, the metallurgical, electronic, and chemical industries, and waste disposal and automobile exhaust. Earlier studies have quantified deposition of metals in the vicinity of the highway or traffic dense area, either by measurement by dry depositions fluxes at various distances from road, or by calculating soil and vegetation concentrations and assuming that the soil acts as long term store, hence effectively integrating the deposition[23],[24]. Lead concentrations as high as 6835, 1180 and 682 ppm dry weight have been reported in soil, vegetation and invertebrates, respectively[25],[23],[24]. The concentrations of toxic trace metals in the soil sample collected from Jaipur, India, have been determined using Direct current Polarography (DC- Polarography). The heavy elements quantities in digested soil samples were as follows: Pb about 7.70 ppm, Cd 7.35 ppm, Ni 2.41 ppm and Zn 2.68 ppm[26].Metal depositions are associated with a wide range of sources such as small scale industries (including battery production, metal products, metal smelting and cable coating industries) ; brick kilns ; vehicular emissions ; re-suspended road dust and diesel generator sets. These can all be important contributors to the contamination found in vegetables. In general, coal combustion is an important source, because Indian coal is of relatively poor quality and has high heavy metal contents. Additional potential sources of heavy metals in field locations in urban and peri-urban areas include irrigation water contaminated by sewage and industrial effluent leading to contaminated soils and vegetables. Other sources can include unsafe or excess application of (sometimes banned) pesticides, fungicides and fertilizers such as sewage sludge[27].Metals belong to the group of foreign materials that are excreted into bile and their ratio of concentration in bile verses plasma is greater than 1.0 and may be as high as 10 to 1000. Since liver is in a very advantageous position for removing toxic materials from blood after their absorption, it can prevent their distribution to other parts of the body. Furthermore, because the liver is the main site of biotransformation of toxic agents the metabolites may be excreted into bile[28]. Lead is absorbed in gastrointestinal tract by two steps process. It is first absorbed from lumen and then excreted into the intestinal fluid[29]. Upon oral ingestion about 5 to 10 % of lead is absorbed and usually less then 5% of what is absorbed is retained[30]. Thus about 99.5 % of total ingested lead is excreted through feces. Out of this 90% is coming out without being absorbed and 9.5% after being absorbed and metabolized leaving only 0.5% to be deposited in various body tissues. Fecal matter analysis method’s distinct advantages over tissue analysis are that the exposure can be measured on daily basis , it does not involve killing or even disturbing the wild mammals, it represents the metal eliminated which has been incorporated due to gross exposure (inhalation, ingestion or dermal exposure) in a locality. Thus, it can be concluded that wild mammals housed in Jaipur zoo are exposed to metallic pollution (air and water). Our study has firmly established the value of fecal matter analysis as bioindicator of heavy metal contamination. At least our study holds out a promise where scat can be used, since it does not involve either disturbing or killing of an animal, as useful bioindicator. The study can be further extended to free-ranging wild animal which are exposed to contaminants that are emitted by vehicles plying on roads within the protected areas.
|
|
4. Conclusions
- Our results shows that fecal matter can be used as good bio-indicator for gross metal exposure and it provides a less expensive or better means of assessing long-term trends in pollution or other forms of environmental change. This method is completely non-invasive one to conserve the wildlife.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML