-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
World Environment
p-ISSN: 2163-1573 e-ISSN: 2163-1581
2013; 3(2): 37-44
doi:10.5923/j.env.20130302.01
Hydrodynamics and Partitioning of Selected Heavy Metals in Surface and Subsurface Soil
Olatunde S. Olatunji1, Olalekan S. Fatoki1, Bhekumusa J. Ximba1, Beatrice O. Opeolu2
1Department of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, Cape Town, South Africa
2Department of Environmental and Occupational Studies, Faculty of Applied Sciences, Cape Peninsula University of Technology, Cape Town, South Africa
Correspondence to: Olatunde S. Olatunji, Department of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, Cape Town, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The transport of cadmium (Cd), lead (Pb), nickel (Ni) and zinc (Zn) in acid rain leachate, through porous surface soil and subsurface soils were investigated in column experiment. The granullometric and geotechnical properties of the different soil horizons were determined using the American Society for Testing and Materials (ASTM) methods. The physico-chemical properties and heavy metal concentrations were determined using standard methods. Surface and subsurface soil horizons, in replica of the profile pit dimension measured at Ilupeju village study site, were packed in 8 x 120 cm polypropylene plastic material. About 250 ml of contaminant solutions containing 100 mg l-1 each of Cd, Pb, Ni and Zn were leached by saturating the column with simulated acid rain until leachates were collected from each soil horizon outlets. The leachates were digested using standard methods and the levels of Cd, Pb, Ni and Zn were determined using flame atomic absorption spectrophotometer. The concentrations of Cd, Pb, Ni and Zn in the acid rain leachates of soils collected at the end of the top soil column were: 18.40±2.57, 0.5±0.12, 7.43±2.19 and 23.02±4.09 mg l-1 respectively. Significant (p<0.05) levels of Cd, Ni and Zn were attenuated in the fourth soil horizon, with leachate concentration decreasing from 9.60±1.58 to 1.03±0.45; 6.66±2.15 to 0.69±0.37 and 11.80±2.26 to 2.60±0.97 mg l-1 respectively, in leachates collected at the column end of the fifth horizon, while Pb concentrations was less than 0.10 mg l-1. The surface and subsurface soil column down to the fourth horizon demonstrated high metal sequestration capacity, hence there may be significant reduction or cessation in the transport of heavy metals to the deeper subsurface soil.
Keywords: Top Soil, Subsurface Soil, Heavy Metals, Contamination
Cite this paper: Olatunde S. Olatunji, Olalekan S. Fatoki, Bhekumusa J. Ximba, Beatrice O. Opeolu, Hydrodynamics and Partitioning of Selected Heavy Metals in Surface and Subsurface Soil, World Environment, Vol. 3 No. 2, 2013, pp. 37-44. doi: 10.5923/j.env.20130302.01.
Article Outline
1. Introduction
- Soils are natural repository and sinks for heavy metals[1]. Their concentrations can be elevated by deposition from anthropogenic sources. Pollutants are subject to different fate upon release on soils; hence they may not remain in their original form and concentration at deposition for long. The transport and fate of contaminants in surface and subsurface soils are related to the nature of the contaminants, soil physico-chemical properties, and the relative abundance of the soil reactive mineral phases. These are considered as the primary controllers of sorption processes in soils, thus serving as important regulators of contaminant transport[2]. Ecosystems tend to respond to the presence of pollutants in different ways[3]. Inorganic pollutants such as heavy metals and some organic pollutants such as pesticides, are persistent and may remain as undesirable residues in food; environment and living tissues; volatilize into air and subjected to long range transport; undergo microbial degradation which enhances their solubility; and or subject to horizontal and vertical transport in soil, especially to the rooting zone[4]. The attenuation of pollutants in soil is a natural phenomenon, and this is related to the transport of contaminants[5]. The bulk mineralogical assemblage of surface and subsurface soils could provide basis for predicting natural attenuation processes and contaminants behaviour in surface and subsurface environments[2]. According to Castrignano et al.[6], physical, chemical and biological processes which are able to reduce the contaminant concentrations could be an effective soil remediation strategy. Heavy metals in soils are subject to a number of soil processes which either result in soil stabilization or reduction, depending on soil conditions and climatic variables[7]. The risk associated with heavy metals arises from their bio-toxic properties and non-degradable nature unlike organic pollutants. Their characteristics water solubility, tendency to adsorb to the soil, persistence and soil granullometric and physico-chemical properties are important in determining the fate of the chemicals in the environment. There is the likelihood of metals partitioning variation in the different soil textural classes, and into different forms in soil. Nielsen et al.[8], McGrath and McCormack[9], and Peijnenburg and Jager[10] reported that, metal transport as well as distribution of metals in soil should take into account the totality of retention of metals in all the soil chemical phases. In this study, the transport and hydrodynamics of some selected heavy metals in acid rain was investigated in surface and subsurface soil in column experiment. The study also examined the effect of some soil physical and chemical properties affecting transport of metal pollutants.
2. Materials and Methods
2.1. Study Area
- The study site with geo-reference coordinate N 07.38700o and E 003.71855o is located in a fallow farmland near Ilupeju village, along Abeokuta-Ibadan Road, Ido Local Government Area, Oyo State (Figure 1). Ilupeju is essentially a farming settlement characterised largely by subsistent crop production, although cassava and maize are commercially produced. The study site is about 500 m away from Abeokuta-Ibadan transit road, with low human activity. An economic deposit of sand buried in the earth along the perennial stream traversing behind the community is located to the southern flank of the study site. The sand deposit is largely exploited for commercial and economic gains.
2.2. Profile Pits
- Five profile pits of about 120 cm depth were randomly dug at distances of about 200 m apart in a circular pattern. The profiles of the soils were studied and the variation in soil texture and type making up the subsurface were noted. Each of the soil horizon column thickness was measured.
2.3. Sample Collection and Preparation
- Soil samples were separately collected from surface and each of the subsurface soil horizons in different nitric acid pre-treated cotton sacks. Core soil samples were also collected from the surface and the different subsurface soil horizons, using thick walled cylindrical cores (8 x 12 cm) of known volume, driven vertically down under pressure into the different soil horizons, for bulk density and hydro-geotechnical properties. Soil samples for physico-chemical and soil residence metal levels analysis were collected in nitric acid pre-treated polypropylene bags. The soils collected for physico-chemical and metals determination were air dried in the laboratory for 96 hours. Portions of the dried soil were then pulverized and sieved using 63 µm sieve size.
2.4. Grain Size Distribution and Textural Characterisation
- Grain size analysis was conducted on soil samples collected from each of the different soil horizons, comprising of the surface and four subsurface soil horizons. 250 g of the soil samples were loaded on integration of graduated sieves and mechanically agitated to facilitate the segregation of the relative proportions of each size range as described in ASTM No D422[11]. Hydrometers and sedimentation cylinders were later used to determine the proportion of fine grained material (i.e. silts and clays). The soil textural classes were identified from textural triangle
 | Figure 1. Field study site off Abeokuta-Ibadan Road, Ido Local Government Area Oyo State (Google maps) |
2.5. Bulk Density
- The bulk or in-place density (expressed by mass per unit volume) of the surface and subsurface soil horizons were determined using the undisturbed soil samples collected in the drive-cylinder as described in ASTM D2937[12]. The soils collected in the core were transferred into pre-weighed evaporating dishes and weighed. They were afterwards dried in hot air oven at 100○C for 2½ hours. The dried core soil samples then were cooled in desiccators, and weighed. The ratios of the weight of soil to the volume of the cylindrical core were calculated.
2.6. Packing Density
- The packing density of the experimental column was evaluated by modifications of ASTM D2937[12]. A cylindrical core made from the experimental column material was packed with soil from the top soil stratum. The packed column was weighed to determine the weight of the packed soil. This was repeated for the subsurface soils from the different horizons. The diameter and length of the column was measured to calculate its volume. The packing density of each of the packed soil horizon were obtain by calculating the ratios of the weight of the soil filled in the column, to the volume of the column core covered by the packed soils.
2.7. Hydraulic Conductivity
- The constant head method which uses a core with constant head tank was used. A plastic core was sealed on one end by tying a cotton fabric material which serves as a porous membrane to it. This was then filled to the brim with soil samples, and introduced into a bow about ¾ filled with water. The soil in the core was allowed to absorb water from the bow until saturated. The saturated core was left for two days to ensure total core saturation, at the instance of which water level in the bowl remain constant. The core containing the saturated soil was extended by attaching an additional plastic core to it in such a way as to avoid leakage at the joint of attachment. Another porous membrane or plug was then placed over the saturated soil, with water poured into it from a specific height. The upper portion of the core was attached to the constant head tank, and the core removed in a bowl clamped over a funnel placed on a tripod stand. The water was allowed to percolate the soil in the core and the drain collected in measuring cylinder. The time taken to collect specified volume of water was taken using a stop watch. This was repeated until the constant time was measured for at least three consecutive collections. The procedure was repeated for soils from each horizon, after which the soil horizons hydraulic conductivities K, permeability and porosity were determined
2.8. Determination of Soil pH
- Slurries of the top soil and subsurface soil horizons were prepared by weighing 5 g each of the soils into 100 ml beakers, and adding 50 ml distilled water into each of them. They were thoroughly mixed and allowed to stand for 30 min. The pH of the surface and subsurface soils were thereafter measured by inserting the electrode of a pre-calibrated pH meter into supernant solutions collected on the top of soil sediments in the beaker[13].
2.9. Residual Analysis
- The background concentrations of heavy metals in the surface and subsurface soil horizons were determined according to the method of Onianwa[14] and Smejkalova et al.[15]. About 5 g each of pulverized soil samples from each of the soil horizon (in triplicates) were digested in 50 ml 2 M nitric acid. The resulting solutions were heated in a water bath at 80○C for two hours. The resulting digest solutions were filter and made up to 50 ml mark in different volumetric flask. The digested samples were quantified for Cd, Pb, Ni and Zn using flame atomic absorption spectrometer (FAAS).
2.10. Construction and Packing of Experimental Column
- Experimental plastic columns of 9.5 x 150 cm (diameter by length) were constructed using polyethylene materials. Water collection outlets were constructed at points representing the column end of each horizon as measured from the profile pit, while the bottom end of the experimental column was made of perforated plastic material to hold the packed column. The experimental column was packed with corresponding profile soils as collected from the different soil horizons (surface and subsurface soils down to the fifth horizon). Each of the columns was compacted to the field dimension, to a total column length of 118 cm.
2.11. Preparation of Simulated Acid Rain
- Acid rain was simulated by acidifying carbonic acid with acid mixture of 3 M sulphuric acid and 3 M nitric acids in ratio 1:1. Carbonic acid was produced by the dissolving the carbon dioxide produced in the reaction between dilute hydrochloric acid with sodium hydrogen carbonate in water. The carbonic acid solution produced was further acidified by dropping the 1:1 mixture of sulphuric and nitric acid until about pH 2.5.
2.12. Soil Treatment and Leaching of Metal Using Simulated Acid Rain and Deionised Water
- Contaminant solutions containing 100 ppm Cd, Pb, Ni and Zn were prepared by dissolving CdCl2, PbNO3, NiCl2 and ZnCl2 salts in 250 ml. The 250 ml, 100 ppm contaminants solutions were applied to the top of the experimental soil columns and allowed to drain down. Simulated acid rain of pH 2.5 was used to leach the metals from the top of the column until leachates were collected from outlets at the end of each soil horizon column.
2.13. Analysis of Acid Rain Leachates
- Leachates collected from column end of each of the soil horizons were digested according to the method described by APHA[16]. 10 ml each of the acid leachates were mixed with 100 ml distilled water. The resulting mixture was heated at a temperature of 85○C to about 10-15 ml volume. The resulting solution was filtered and made up to 50 ml mark in a plastic volumetric flask. The digested leachate solutions were quantified for Cd, Pb, Ni and Zn using flame atomic absorption spectrometer (FAAS).
3. Results and Discussion
3.1. Surface and Subsurface Soils Resident Concentration Levels of Cd, Pb, Ni and Zn
- The evaluation of the natural resident level of the heavy metals in soil provides the basis for understanding the interaction, retention and transport characteristics of the pollutant heavy metals in porous soils. The concentrations of heavy metals in the surface and subsurface soils collected from different horizon are presented in Table 1. The concentration of Cd, Pb, Ni and Zn were highest in the top soils with mean values of 0.64±0.02, 10.07±1.62, 4.83±1.27 and 14.27±3.02 mg kg-1 respectively. The concentrations in the different subsurface horizons were variable and were not of any definite trend. The lowest subsurface concentrations of Cd, 0.35±0.02 mg kg-1 was detected in horizon-4; Pb, 3.15±1.05 mg/kg in horizon-2, while the least concentration of Ni, 2.81±1.03 mg kg-1 and Zn, 4.15±1.90 mg kg-1 were found in horizon-5. The detected levels may be of natural geochemical origin, and are within EEC guideline levels recommended for arable land[17].
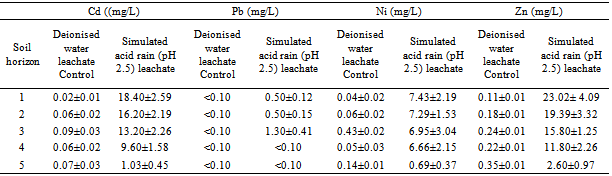
3.2. Concentration of Cd, Pb, Ni and Zn in Leachates Collected From Horizon Column Interfaces of the Surface and Subsurface Soils
- The result of the determination of the concentrations of Cd, Pb, Ni and Zn in the acid rain leachates and the deionised water leachates (which served as experimental control) collected at the column interfaces between the soils horizons, from top soil down to the subsurface are presented in Table 2. The concentrations of the heavy metals were significantly (p<0.05) higher in the acid rain leachates collected at column end of the top soil than in leachates collected from strata interfaces of the subsurface soils down to a depth of 118 cm.The result showed that significant amount of the metals spiked on the surface soil were retained within soil matrices of the top four horizons with combined thickness of 52.50 cm. The concentration of the heavy metals decreased in acid leachates as it percolates and drain through the porous packed experimental column. The concentrations of Cd, Pb, Ni and Zn in the simulate acid rain soil leachates collected at the end of the top soil, with column thickness 7.80-9.61 cm were 18.40±2.57, 0.5±0.12, 7.43±2.19 and 23.02±4.09 mg l-1 respectively. Significant (p < 0.05) amount of Cd, Ni and Zn were attenuated in soil horizon-4, with leachate concentration decreasing from concentration 9.60±1.58 to 1.03±0.45; 6.66±2.15 to 0.69±0.37 and 11.80±2.26 to 2.60±0.97 mg l-1 respectively, in leachates collected at the column end of the fifth horizon. The concentrations of Pb in acid rain leachates collected from column end of soil horizons-1, -2 and -3 were low and ranged 0.50±0.15 - 1.30±0.41 mg l-1, while Pb concentration in leachate from horizon-4 and horizon-5 were below instrument detection limit (< 0.10 mg l-1). The lowest concentrations of Cd, Pb, Ni and Zn were observed in leachates collected at the column end of horizon-5, with concentrations 1.0.3±0.45, <0.10, 0.69±0.37 mg l-1 and 2.60±0.97 mg l-1 respectively. This suggests that there is low tendency for deep subsurface contamination by pollutant heavy metals, because substantial proportions of the heavy metal spikes were sequestered within the first four soil horizons. However the concentration of the measured metals in distilled water leachates (experimental control) were low and ranged; Cd, 0.02±0.01 - 0.09±0.03 mg l-1, Ni, 0.05±0.03 – 0.43±0.02 mg l-1, and Zn, 0.11±0.01 - 0.35±0.01 mg l-1, while Pb was not detected (<0.10 mg l-1). Large fractions of the spiked metals were retained on the top soil in the control column, while acidity facilitated the transport of metals down through the fourth soil horizon. The transport of the heavy metals in distilled water leachates is low compared to acid rain leachate. Most of the spiked metals were retained on the top soil in the control column, while acidity facilitated the transport of metals down through the fourth soil horizon. Lead was relatively immobile compared to other metals. The acidity of the simulated acid rain as well as the soil acidity did not appear to enhance the mobility of Pb. The mobility of Zn was the highest with concentrations 23.02±4.09 mg l-1 in the simulated acid rain leachates collected from the first horizon (8.75) and 2.60±0.97 mg l-1 in the fifth horizon (118 cm) respectively. Significant amount of Zn was retained within the first four soil horizons. The mobility characteristic of Cd was similar to that of Zn. There was gradual decrease in leachate concentration of Cd, from 18.40±2.59 in the first horizon to 9.60±1.58 mg l-1 in the fourth horizon. The fourth soil horizon attenuated significant amount of Cd from 9.60±1.58 mg l-1 in the fourth horizon to 1.03±0.45 mg l-1 in the fifth horizon. There was no appreciable reduction in concentrations of Ni in leachates collected from the first horizon (7.43±2.19 mg l-1) to 6.66±2.15 mg l-1 in the fourth horizon. Leachate collected at the column end of the fifth horizon, showed that significant concentration of the applied heavy metals; Cd, (98.97%), Pb, (99.9%), Ni, (99.31%) and Zn, (97.4%) were retained within the five horizons. The acidity of the simulated acid rain apparently influenced the hydrodynamic transport/mobility of the metals in comparing the levels of the heavy metals detected in the acid rain leachate with the levels detected the deionised water leachate (control column).
3.3. Physico-Chemical and Granullometric Properties of Surface and Subsurface Soils
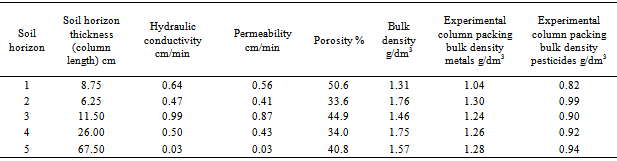
- The ability of soils to retain heavy metals can be attributed to a number of dependent soil granullometric and physico-chemical properties including grain size distribution, soil pH, organic carbon (OC), Fe/Mn oxy-hydroxide and soil cation exchange capacity (CEC)[18]. The column thickness of the top soil (8.75 cm) was the least while the column thickness of subsurface soil horizon-5 (67.50 cm) was the highest. The surface and subsurface soils were generally slightly acidic with varied pH ranged 4.9 – 5.7 (mean pH 5.2±0.3) (Table 3). This could be due to variations in particle size distribution of the surface and subsurface soil horizons, the relative proportion of soil solution present in the pore spaces of the solid soils, variation in the adsorption of cationic H+ on the solid surface and the soil heterogenic composition nature. Schilling et al.[19] reported that the effectiveness of soil minerals at consuming excess acidity in rainwater enhances metal mobilization from these interactions. The cation exchange capacity (CEC) of the surface and subsurface soil horizons varies with values ranged 2.14 – 4.78 µeq/g. The CEC of the surface soil is significantly higher (p < 0.05) than in the subsurface soil. Results showed that surface soil with 1.80% OC, colloidal composition of 5.80% silt and 7.20% clay, and 4.78 µeg/g CEC allowed the leaching of 7.5% Ni, 18% Cd and 23% Zn, while Pb was hardly mobilized (0.5%). The concentration of the measured metals in leachates collected from the second to the fourth soil horizons were not significantly (p>0.05) different from each other. The fifth soil horizon with increased silt and clay composition of 9.80% silt and 12.60% respectively, with OC, 2.14% and CEC of 0.25% retained almost all the metals in leachates reaching it from the fourth soil horizon. Hence leachates from the fifth horizon had Cd, Ni and Zn concentrations of 1.03, 0.69, Ni 2.6 mg/L respectively, while Pb was not detected. Nearly 91% of Cd, 97% Ni and 82% of Zn were retained within the first and the fourth soil horizons in traversing a total column length of 52.50cm. The retention of pollutants metals in soils depend on the magnitude of its permanent charge and pH, which controls the process of protonation and deprotonation of soil organics and clay minerals[20]. Study result indicates that the surface soils are not near neutral, hence the total net charge is not equal to zero. According to Lewis[21], the reactivity of soils depends on the magnitude of the points of zero charge (PZC), which is defined by the nature and charge density of soil particle and soil pH. The pHs of the different soil horizons ranged 4.90 – 5.70, and was within the typical pH of 4 – 9 which governs the mobility of nutrients and contaminants in soils. The release of exchangeable cations in the different horizons may results in reactive soil sorbent surfaces, and partly enhancing the sorption/retention capacity of the soil. Each soil horizon will therefore behaves differently depending on its CEC and prevailing soil pH, with each having its unique interaction with pollutants solutes (metals or organics). Soils with high CEC have stronger interaction with pollutants hence their ability to retain or slow their mobility, during which the process of the degradation of pollutants solutes may take place[22]. Consequently pollutant metals are prevented from deeper subsurface and ground water. Organic carbon in the different soil horizons varied between 0.25 - 1.80%. Organic carbon was reported to play critical role in pollutant mobilization and or transport[23]. Metal are reported to be partly retained by complexation interaction with organic matter. Study result showed higher concentrations of metals in soil horizon rich in OC than those with low OC. Top and subsurface soils of the different soil horizon showed variation in metals complexation effect which depends on the soil OC content, and the soil horizon thickness. Soil OC content partly defines the levels of metals and other pollutant that can be transported in the leaching fluid or soil solution. However, microbial degradation of soil organic matter may reduce the retention capacity of the soil horizons, thereby enhancing the release of pollutants metals in leaching solution. Hence, each horizon is intrinsic in its ability to either retain or trans-locate pollutant metalsGrain size distribution and textural analysis of soils from the different horizon showed that each of the soil strata is predominantly made up of sand, followed by silt and low in clay, thus the nearly uniform bulk density. Soil textural class affects the soil fluidal hydrodynamics and mobility of pollutants. According to Salmasi and Tavassoli[24], the retention of heavy metals in fine clay grain was higher than in sandy or coarse grained soils. This was attributed to the high surface area and charge density of fine grained clay soils. Thus the sorption capacity of soil is partly a function of the relative amount of soil particulate sizes. The sandy nature of the different horizons affects soil hydraulic conductivity, permeability and porosity, and thus the horizontal and vertical flow dynamics in top and subsurface soils.
3.4. Geotechnical Properties of Study Site Soils
- The hydrodynamics in soils depends on the soil geotechnical properties such as bulk density, hydraulic conductivity, permeability and porosity[25]. The bulk density of the topsoil and subsurface soil horizon showed that the second horizon consisting of loamy soil had the highest bulk density of 1.76 g/dm3, while the top a-horizon loamy sand has the least of 1.31 g/dm3. Consequently the infiltration of fluvial materials such as air and water are expected to be rapid in top soil when compared with subsurface soils. The differences in bulk densities of the different soil horizons imply differentials in hydraulic conductivity, porosity and fluid permeability in the surface and subsurface soils. The packing density of soil in each horizon of the experimental column ranged 0.82 – 0.99 g/dm3. This showed a 22±19% distortion from bulk density of the undisturbed soil, in packing the experimental column, and this implies higher hydraulic conductivity, porosity and fluid permeability than may be observed on the field. The measured hydraulic conductivity, porosity and fluid permeability were 0.03 – 0.99 cm/min, 33.6 – 50.6% and 0.03 – 87 cm/min respectively. The bulk (1.76 g/dm3) and packing density (1.30 g/dm3) of subsurface soil horizon-2 were the highest, but with the least hydraulic conductivity (0.47 cm/min), porosity (33.6%) and fluid permeability (0.41 cm/min). Soil horizon-1 had the least bulk density (1.31 g/dm3) and packing density (1.04 g/dm3), and hydraulic conductivity (0.64 cm/min), porosity (50.6%) and fluid permeability (0.56 cm/min). Although, the hydraulic conductivity and fluid permeability of soil horizon-1 was expected to be the highest as a result of its low bulk and packing density, soil horizon-3 had the highest hydraulic conductivity (0.99 cm/min) and fluid permeability (0.87 cm/min). Hence, the nature of pollutants and their inter relationship with surface and subsurface soils determines the mechanism and transport of heavy metals through porous soil media. Therefore the mobility or leaching of heavy metals in soils depends on whether the pollutants exist as reactive or non-reactive species and the magnitude of soil reactive functional sites. Reactive pollutant species are more likely to be retained in soils as they are held bonded to leachate insoluble chemical substances in the soil, while the non-reactive species and neutral pollutants are transported in the leachates.
|
|
|
|
4. Conclusions
- The relative mobility of the metals through the soil are in the order Zn > Cd > Ni > Pb. The geophysical, hydro-geochemical and physico-chemical properties of soils were noted to have varying degree of effect on the mobility and adsorption of trace metals. The surface and subsurface soil column down to the fourth soil horizon demonstrated a high capacity to retain Cd, Ni and Zn, while Pb was largely attenuated on the top soil. The mobility of Zn was pH influenced and found to be the highest, while Pb was the least mobile. The retention of metals on surface and subsurface soils may be associated with high OC content and the soil mineral assemblage, with the top soil being higher. Thus, there may be significant reduction or cessation in concentration of heavy metals reaching the deeper subsurface soil, since metals are strongly retained within the first soil horizon and the fourth soil horizon in the subsurface, though there may be significant long–term risk of metal leachates reaching deeper soils and groundwater.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML